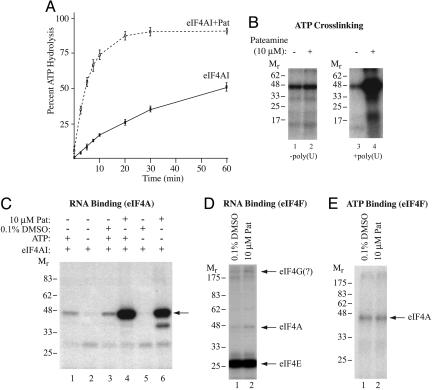
Fig. 3.
Pateamine stimulates eIF4AI activity. (A) Pateamine stimulates eIF4AI-mediated ATP hydrolysis. ATPase assays were performed for the indicated times by using 1 μM γ-[32P]ATP/1 μM poly(U)/3.6 μg of recombinant eIF4AI and monitored by thin-layer chromatography. Quantitations were performed by using Fujix BAS2000 with a Fuji imaging screen, and the data are from a total of three experiments. (B) Pateamine stimulates ATP binding to eIF4AI in the presence of RNA. Crosslinking of ATP was performed with 1 μg of recombinant eIF4AI and 2.5 μCi (Ci = 37 GBq) of α-[32P]ATP (3,000 Ci/mmol) by using UV light and resolved by SDS/PAGE. The presence or absence of 7.5 μM poly(U) is indicated below. The gel was dried and exposed to x-ray film (Kodak) at -80°C for 12 h with an intensifying screen. (C) Pateamine stimulates RNA-binding activity of eIF4AI. 32P-cap-labeled CAT mRNA (105 cpm) was incubated with recombinant eIF4AI (1 μg) for 10 min at 30°C, chemically crosslinked, treated with RNase A, and resolved by SDS/PAGE. Gels were dried and exposed to x-ray film (Kodak) at -80°C for 1 h with an intensifying screen. (D) Chemical crosslinking of eIF4F to 32P-cap labeled oxidized mRNA. The presence of 10 μM pateamine is indicated at the top. Components of the eIF4F complex are labeled to the right. The gel was dried and exposed to x-ray film (Kodak) at -80°C with an intensifying screen. (E) UV light-induced crosslinking of α-[32P]ATP to eIF4Ac. Crosslinking of ATP was performed with 0.74 μg of eIF4F and 2.5 μCi of α-[32P]ATP (3,000 Ci/mmol) by using UV light and resolved by SDS/PAGE. The gel was dried and exposed to x-ray film (Kodak) at -80°C.