Abstract
Recently, 3-D porous architecture of the composites play a key role in cell proliferation, bone regeneration, and anticancer activities. The osteoinductive and osteoconductive properties of β-TCP allow for the complete repair of numerous bone defects. Herein, β-TCP was synthesized by wet chemical precipitation route, and their 3-D porous composites with H3BO3 and Cu nanoparticles were prepared by the solid-state reaction method with improved mechanical and biological performances. Several characterization techniques have been used to investigate the various characteristics of fabricated porous composites. SEM and TEM studies revealed the porous morphology and hexagonal sheets of the β-TCP for the composite THC8 (82TCP-10H3BO3-8Cu). Moreover, the mechanical study showed excellent compressive strength (188 MPa), a high Young’s modulus (2.84 GPa), and elevated fracture toughness (9.11 MPa.m1/2). An in vitro study by MTT assay on osteoblast (MG-63) cells demonstrated no or minimal cytotoxicity at the higher concentration, 100 µg/ml after 24 h and it was found a more pronounced result at 20 µg/ml on increasing the concentration of Cu nanoparticles after incubating 72 h. The THC12 composite showed the highest antibacterial potency exclusively against B. subtilis. S. pyogene, S. typhi and E. coli. at 10 mg/ml, indicating its potential effectiveness in inhibiting all of these pathogens. Genotoxicity and cytotoxicity tests were also performed on rearing Drosophila melanogaster, and these findings did not detect any trypan blue-positive staining, which further recommended that the existence of composites did not harm the larval gut. Therefore, the fabricated porous composites THC8 and THC12 are suitable for bone regrowth without harming the surrounding cells and protect against bacterial infections.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-87988-4.
Keywords: β-tricalcium phosphate, Composites, Morphology, Mechanical performances, In vitro-study, Bone repair, Antibacterial
Subject terms: Biomaterials, Biological techniques, Cancer
Introduction
Bone defects are generally related to infection, accidents, genetic disorders, or trauma1. More than 4.5 million bone defects are observed in India, and more than 70 million bone injuries occur worldwide annually1,2. Although bone defects are of several sizes, the term “critical size” refers to longer defects where the bone is unable to heal spontaneously3. The critical size defects can be healed by employing bone grafting or regeneration.
Numerous synthetic materials, such as metals, ceramics, and polymers, have been suggested for use as bone grafting materials4–6. Hard tissues contain a mineral component made up of calcium-deficient (C-D) apatite7. Nevertheless, calcium phosphate ceramics have received the greatest research attention and are generally used in orthodontics and orthopaedics, including hard tissue engineering. Owing to the above mentioned applications, the hydroxyapatite (HAp, Ca10(PO4)6(OH)2) and tri-calcium phosphate (TCP, Ca3(PO4)2) have been painstaking as the greatest viable inorganic compounds for replacing bone tissues8,9. In the family of calcium phosphates, β-TCP is widely used for hard tissue regeneration owing to its biocompatibility and biodegradability10. Its osteoinductive and osteoconductive properties allow for the complete repair of damaged bones in addition to its cell-mediated resorption11. Although, β-TCP has a greater bone resorption rate than that of HAp, this rate is lower than the rate of newly bone formation, and the resorption rate of β-TCP may be accelerated by either reducing particle size or dopants12–14. Despite, its excellent biological properties, calcium phosphate and its derivatives (HAp, and TCP) face significant limitations in practical applications and challenging due to its poor mechanical characteristics, including high brittleness, low fracture toughness, and poor wear resistance15. To address these limitations and make material suitable for implantation by incorporation of appropriate metal, oxide, or carbide phases into the base materials16. Researchers have explored the potential of TCP ceramics as drug carriers for various therapeutic agents, including antibiotics, proteins, anticoagulants, and anti-cancer drugs. Nevertheless, the slow or irregular rapid rate of the degradation in tissue fluids has restricted the widespread use of TCP as drug delivery systems17,18. Moreover, the enhanced functional properties, including drug carrier, anticancer, and antibacterial activity19,20 of TCP can be obtained by using numerous dopants like copper (Cu), zinc (Zn), silver (Ag), gold (Au), iron (Fe), or carbon nanotube (CNT)21–24. Recently, Harb et al.25 and Hu et al.26 prepared scaffolds of the composites PLA/TCP/ZnO and TCP/TiO2 by the 3-D printing method for the improvement of mechanical behaviours, bone tissue engineering, and antibacterial properties. Amaral et al.27 fabricated a β-TCP-based composite with bioactive glass (S53P4) to increase bone formation and antibacterial activity. Wang and colleagues were reported that the immunogenic activities of HTB-loaded Zn- and Mg-TCPs were assessed both in vitro and in vivo to determine their potential as immunopotentiating adjuvants for cancer immunotherapy28. The anticancer properties of β-TCP and its composites were investigated by few researchers yet. Moreover, the increasing number of mortality related to antibiotic resistance and cancer has led to a strong demand for materials having inherent anti-tumor or antibacterial properties29,30. Antibiotic susceptibility to several drugs is a stealth pandemic that mostly affects vulnerable groups, including the elderly, the immunocompromised, and the polypathologic. The widespread use of broad-spectrum antibiotics in patients with numerous bacterial infections has greatly exacerbated the condition. In order to curtail or eradicate the overuse of antibiotics, the fabrication of novel materials possessing antibacterial qualities is crucial and challenging31,32.
In the present study, a ternary composite has been made using boric acid (H3BO3), Cu nanoparticles, and pure β-TCP. Boric acid has the potential to be a promising cancer treatment agent, demonstrating the ability to curb cell growth and trigger programmed cell death. Barranco and Williams revealed that boric acid has the capability to both slow the proliferation of prostate cancer cells and initiate their apoptosis33,34. Meacham et al. found that boric acid increased the effectiveness of some chemotherapy drugs while simultaneously decreasing the viability of breast cancer cells, which lends further weight to these findings35.
Gölge et al. investigated the effects of boric acid on fracture healing using an animal model using 40 male Sprague-Dawley rats. Their findings revealed significant differences in average histological scores between the groups, with treated groups showing notably higher scores as compared to the control groups. These results suggest that boric acid may have potential as a therapeutic agent for enhancing fracture healing process. However, Singh et al. suggested that supplementation with boric acid equivalent to 200 ppm boron (B) positively influenced bone formation by increasing plasma levels of bovine alkaline phosphatase, osteocalcin, 25(OH)-vitamin D3, and calcium. At the same supplementation level, boric acid also inhibited bone resorption, as specified by lower plasma N-telopeptide concentrations, without affecting parathyroid hormone (PTH) or calcitonin levels. Further, Ulu et al. demonstrated that boric acid enhances new bone formation during the early stages of the bone healing process when administered either locally or systemically36–38. H3BO3 also revealed antifungal, antiseptic, or non-toxic properties and was used to create the pores in the composites at higher temperature39,40. The choice of Cu nanopowders as a dopant within TCP is mainly attributed to their outstanding antibacterial and anticancer properties41. However, copper exhibits robust membrane penetration capabilities and intracellularly, it emulates peroxidases within Fenton’s reaction in acidic environment42. Consequently, it induces the production of reactive oxygen species (ROS), creating a cascade that can degrade the typical structure of bacterial cells. The microenvironment of tumors is characterized by mild acidity and an elevated levels of glutathione and hydrogen peroxide43. Consequently, it is anticipated that copper ions will produce a large concentration of oxidizing hydroxyl radicals in the tumor’s environment and cause tumor cell death. Furthermore, in order to increase the osteogenic potential of the materials, the released copper ions can stimulate osteogenic differentiation of stromal cells from the bone marrow44,45. Therefore, it is expected that TCP combined with Cu, which may gradually release copper ions, will accomplish a dual function of stimulating bone growth and anti-cancer treatment. The pure TCP powder was produced using a wet chemical method, while TCP-H3BO3-Cu composites were fabricated via a bottom up approach, i.e. solid-state reaction route. Further, the fabricated composites were studied by several characterization techniques, including XRD, XPS, UV-Vis, SEM, TEM, mechanical properties, and in-vitro cytotoxicity studies for cancer treatment.
Result and discussion
XRD analysis
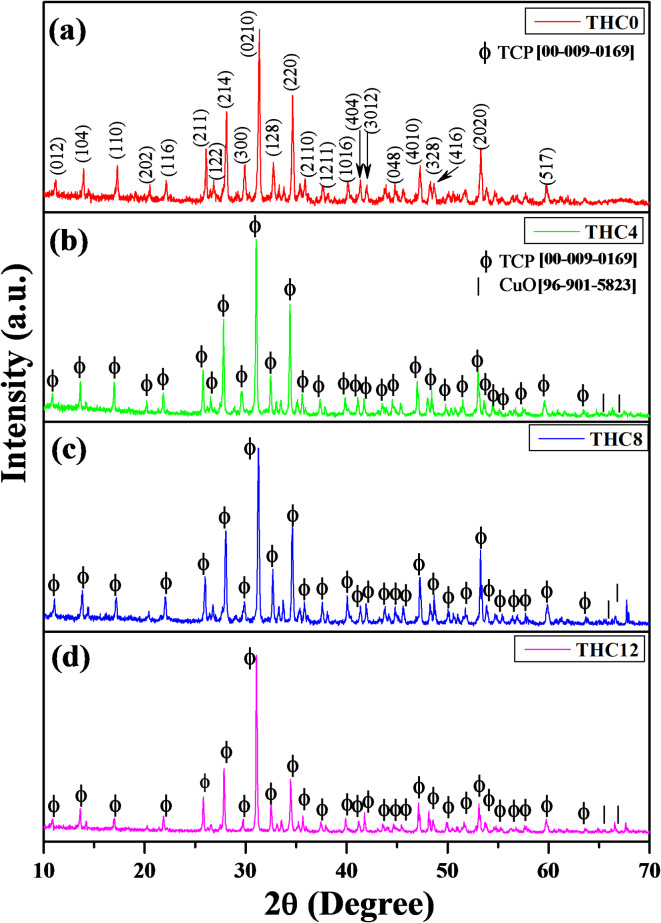
XRD patterns illustrated the polycrystalline characteristics of the synthesized composites THC0, THC4, THC8, and THC12 and all desired crystalline phases depicted in Fig. 1a–d, respectively. The XRD diagram (Fig. 1a) of the composite THC0 revealed a single-phase β-TCP because 10 wt% added H3BO3 into β-TCP vanished after being sintered at 1050 °C for 3 h. Herein, the recorded XRD pattern reflects five characteristic peaks of rhombohedral β-TCP at 2θ values 26.08, 28.1, 31.36, 34.64, and 53.28 corresponding to the lattice planes (211), (214), (0210), (220), and (2020), respectively, and is confirmed by JCPDS file no. [00-009-0169] with space group R-3c (167). On adding 4 wt% of Cu nanoparticles into β-TCP/H3BO3 composites (i.e., THC4), it revealed β-TCP (Fig. 1b) as a primary phase and copper oxide (CuO) as a secondary phase, which are manifested by the symbols phi (ϕ) and star (★), respectively. And CuO was verified by JCPDS file no. [96-901-5823] with a monoclinic structure and space group C2/c. Figure 1c, d displays similar patterns with increased intensity of CuO on increasing the wt% of Cu nanoparticles up to 8 wt% (THC8) and 12 wt% (THC12). The average crystallite size and crystallinity (%) of all nanocomposites were determined in the range of 48.79 –83.85 nm and 66.28 − 66.77%, respectively, and listed in Table 1. Additionally, Match software was used for theoretical investigation (Fig. S3). The outcomes of this investigation strongly agreed with the experimental XRD studies of every prepared nanocomposite. The crystal structures of the major and minor phases were created using VESTA software. There are 63 Ca-atoms (green colour ball) and 42 PO4 groups (P-atoms are represented by golden balls, and O-atoms are denoted by red balls) contained in one-unit cell of β-TCP (Fig. 2a, CIF no. 1517238), and the Ca-atoms were located at 5 distinct Ca positions. The unique structure of β-TCP is that a Ca atom only occupies 50% of the six Ca(4) positions46–48. Jay et al. determined that the half occupancy of fractional Ca(4) is not random but rather repeats in a pattern of 1/3 and 2/3 along the c axis using a pair potential model, remarkably indicating the accurate occupancy arrangement may change due to their simulations, which only deliberated a limited number of computational options. They advised that the crystal structure might be made up of several tiny ordered zones that might seem random to XRD48. Figure 2b displays the unit cell structure of CuO (CIF no. 9015924), containing six Cu-atoms (blue colour ball) and sixteen O-atoms. A central Cu atom was surrounded by four O-atoms to form a polyhedral structure with a bond length of 2Å of Cu-O.
Fig. 1.
XRD patterns of the synthesized composites (a) THC0 shows the single phase of β-TCP, (b) THC4, (c) THC8, and (d) THC12 represent β-TCP as a major and CuO as a minor phase.
Table 1.
Various parameter of synthesized composites including average crystallite size, percentage of crystallinity, average grain size, average particle size and optical band gap.
| Sample’s name | Average crystallite size (nm) | Percentage of crystallinity | Average grain size (μm) | Avg. particle size (nm) | Direct band gap (eV) |
|---|---|---|---|---|---|
| THC0 | 48.79 | 66.53 | 4.10 | 615 | 4.401 |
| THC4 | 65.71 | 66.28 | 3.87 | 531 | 4.370 |
| THC8 | 71.46 | 67.77 | 3.23 | 459 | 4.427 |
| THC12 | 83.85 | 66.45 | 2.97 | 396 | 4.453 |
Fig. 2.
Crystal structure of TCP (a) and copper oxide (b), these structures were created by VESTA.
FTIR analysis
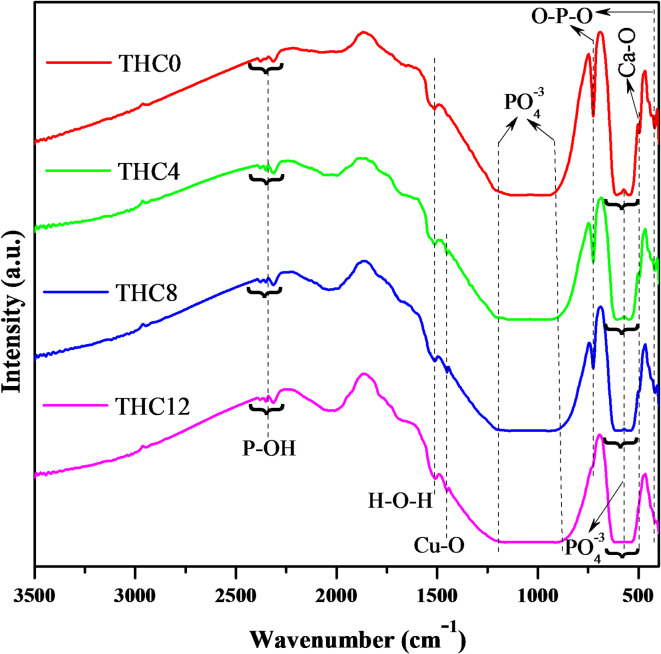
Figure 3 represents the FTIR spectra of all prepared composites. The low wavenumber transmittance peaks at 418 cm−1, and 420 cm−1 might be assigned to the symmetric bending vibration of O-P-O bonds49, while the peaks at 496 cm−1 and 497 cm−1 revealed stretching vibrations of Ca-O bond50. The doublet transmittance peaks arose by O-P-O asymmetric bending of PO4 − 3 in the wavelength range of 540 cm−1–630 cm[−149,50, while a sharp peak has been observed at 725 cm−1 due to symmetric stretching of P-O-P linkage, and it decreases with increasing the wt% of Cu nanoparticles51,52. The wide transmittance peaks were also ascribed by P-O asymmetric stretching of phosphate group within a broad wavelength range from 881 cm−1 to 1205 cm[−149. Near the mid-range of wavelength, a very weak peak at 1450 cm−1 was attributed to Cu-O vibration after adding the 4 wt% of Cu nanoparticle into TCP, and it increases gradually with increasing the amount of Cu53. The peaks about 1510 cm−1, 1512 cm−1, and 1514 cm−1 can be attributed to H-O-H vibrations of water content in atmosphere54. However, the numerous transmittance peaks have been observed at higher wavenumbers, including 2314 cm−1, 2352 cm−1, 2378 cm−1, and 2380 cm−1 due to P-OH vibrations55 and listed in Table S1.
Fig. 3.
FTIR spectra of prepared composites THC0, THC4, THC8, and THC12 sintered at 1050 °C for 3 h.
UV-visible spectroscopic analysis
The UV-visible absorbance spectra (250–900 nm), along with Davis and Mott plots for the indirect band gap, are presented in Fig. 4a and b for the synthesized TCP and their composites, THC0, THC4, THC8, and THC12, sintered at 1050 °C for 3 h. The UV-visible spectra indicate significant absorption in the UV region for each composite, with no observable absorption in the visible region. Figure 4a demonstrates that while there is minimal absorption in the UV region, the plot becomes flat in the visible region. This characteristic suggests that the fabricated composites hold potential in the field of biomedicals. The optical energy band gap values were determined for pure TCP and its composite samples using Tauc’s plot via Eq. (1). The optical band-gaps were measured within a range of 4.427–4.453 eV. Specifically, the minimum concentration of Cu, i.e., undoped TCP, resulted in an optical band gap of 4.401 eV, whereas the highest concentration of Cu showed a band gap of 4.453 eV. This trend reveals a decreasing band gap of the composites with increasing amount of Cu up to 4 wt% of Cu, Further, at 8 and 12 wt%, it was increased (Table 1). The estimated energy-band gap values also suggest that the prepared composites are insulators in nature, thus it can be safely used in biomedical fields.
Fig. 4.
(a) UV-Visible absorption spectra, (b) Tauc’s plot, (c) Skin depth, and (d) particle size of all synthesized composites THC0, THC4, THC8, and THC12.
Additionally, the calculated skin depth (δ) using Eq. (2) led to the plotting of a graph depicting δ versus photon energy (hυ), as illustrated in Fig. 4c. Consequently, the determined values for the cut-off wavelength (λcut−off) and the corresponding cut-off energy (Ecut−off) were determined as 256 nm and 4.848 eV for all the synthesized TCP composites.
Particle size via DLS technique
The particle size plot is depicted in Fig. 4d. The hydrodynamic diameters of the composite THC0 varied from 531 to 712 nm, and its average particle size was measured 615 nm. Furthermore, the particle size range and average particle size of the composites steadily decrease as the amount of Cu nanoparticles increases from 4 to 12 wt% into TCP/H3BO3 composites. Herein, the amount of Cu nanoparticles determines their interactions, agglomeration behaviour, and the resulting impact on composite characteristics, which is also confirmed by SEM micrographs. The particle distribution of the composites THC4, THC8, and THC12 lies within the ranges of 459 to 615 nm, 396 to 531 nm, and 342 to 459 nm, with corresponding average particle size values found to be 531 nm, 459 nm, and 396 nm, respectively (Table 1).
SEM analysis
To determine the structural variations on the surfaces of composites sintered at 1050 °C for 3 h. The morphologies of the composites THC0, THC4, THC8, and THC12 are illustrated in Fig. 5a–h at 1 K and 5 K magnifications. The SEM microstructure results of TCP, interlinked microstructures, densifications, and pores were observed with an increasing content of Cu nanoparticles. Figure 5a, b illustrates the surface morphology of THC0, revealing interconnected grains along with randomly oriented clusters and TCP sheets marked by red and cyan dotted circles and rectangles, respectively. The average grain size of the composite THC0 was determined to be 4.10 μm. However, the SEM image of composite THC4 is elucidated in Fig. 5c, d, showing interlocked TCP grains (pink dotted circles) and sheets (blue dotted lines). The average grain size decreased (3.87 μm) and accumulated with increasing Cu nanoparticles, along with irregular pores, as clearly observed at high magnification. As the amount of Cu increases (up to 8 wt%), H3BO3 also increases slightly (wt% is constant). This increasing amount of H3BO3 contributes to the formation of pores within the fabricated composites. The micrograph of THC8 demonstrates the highly interconnected grains (Fig. 5e) and overlapped hexagonal sheets of TCP, which are bounded by a cyan-dotted rectangle box (Fig. 5f). The average grain size of THC8 composite was further decreased and determined to be 3.23 μm. The macro-circular pores are clearly visualized in the SEM image of THC8 composite, which is highlighted by spring green dotted circles (Fig. 5e). The maximum compressive strength was noticed for the composite THC8, which resulted from round pores and well interconnected grains. Obada et al.56 proposed that the mechanical behaviour of the materials was significantly influenced by the shape and size of the pores at elevated temperatures. When pores are flattened, they tend to decrease the strength of the materials. Conversely, when pores exhibit round shapes, the strength of the material increases. Furthermore, Fig. 5g, h revealed the surface morphology of the THC12, with numerous clusters formed by small accumulated grains (2.97 μm) and the few grains of copper nanoparticles (amber dotted circles) scattered on the surface. The clusters appearing in THC12 are also calibrated and obtained in the range of 10–22 μm. The flattened pores (white dotted ellipse) are observed in between the agglomerated grains in the composite THC12. The pores become interconnected (flattened), and their shape and size have been altered. In other words, these interconnected pores are clearly visualized as micron-order channels that play a vital role in the flow of the body fluid in the large area responsible for cell adhesion as well as proliferation.
Fig. 5.
SEM images of the synthesized composites at different resolutions; (a, b) THC0 showing the TCP cluster with mixed morphology of different grains size, (c, d) THC4, on introducing Cu the grains were agglomerated, (e, f) THC8, showing irregular pores after increasing the concentration of TiC, and (g, h) illustrating the flattened pores and scattered Cu nanoparticle on the surface of the composite THC12. All the composites were sintered at 1150 °C for 3 h.
Additionally, the average elemental investigation of the composites THC0, THC4, THC8, and THC12 was captured and depicted in Fig. S4a–d. The composite THC0 exhibited highest amount of oxygen (O) (50.95%), calcium (C) (30.35%), and phosphorous (P) (18.7%), confirming the pure phase of TCP composite. Thereafter, the composite THC4 has the maximum amount of O (50.32%), Ca (30.74%), and P (18.13%), while a small quantity of copper nanoparticles (0.81%) has been detected. In the composite THC8, the elements were noticed with a large concentration of O (50.33%), Ca (30.4%), P (18.29%), and Cu (0.98%). Lastly, the composite THC12 showed similar results, with highest content of O (51.85%), Ca (28.89%), P (17.92%), and Cu (1.34%). The elemental studies of all the composites revealed the absence of any foreign impurities on the surface.
TEM analysis
Herein, the composite THC8 was shown to have higher mechanical performance and surface morphology when compared to the β-TCP and its composites THC0, THC4, and THC12. Therefore, the TEM study was carried out to investigate the particle size and morphology of the composite THC8 in more detail. Figure 6a shows a TEM micrograph of THC8, which shows hexagonal sheets of TCP. At a low magnification, the TEM micrograph (Fig. 6b, c) reveals a similar porous morphology as illustrated in SEM analysis (Fig. 5e); the pores are widely distributed within the well interconnected TCP grains, as revealed in Fig. 6b. The average pore diameter of the composite THC8 was calculated as 0.156 μm. An HR-TEM image of the prepared composite (Fig. 6c) shows lattice fringe patterns; the interplanar spacing of 0.25 nm and 0.28 nm corresponds to the lattice planes (0210) and (131) of JCPDS card no. 00-009-0169, confirming the major contribution of TCP. Further, to understand the structure of the synthesized composite, a selected area electron diffraction pattern (SAED) was employed. The SAED pattern shown in Fig. 6d clearly shows the polycrystalline pattern, and the diffraction spots (110), (116), (1010), (125), (0210), (131), (404), and (3018) correspond to the major phase of rhombohedral TCP, and (421) corresponds to the minor phase of monoclinic CuO. These diffraction spots were in complete agreement with XRD results.
Fig. 6.
(a) TEM image of THC8 composite, (b) TEM image showing presence of macro-pores, (c) HR-TEM image showing presence of fringe-pattern of the synthesized composite THC8, and (d) polycrystalline SAED diffraction pattern.
XPS analysis
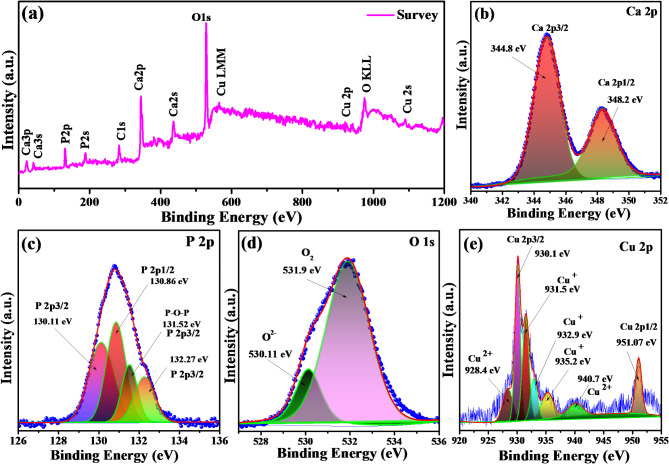
The survey XPS spectra of the porous composite THC8 are illustrated in Fig. 7a and revealed the purity of the composite by elemental analysis and were in well agreement with EDAX outcomes. The XPS spectra display the emission of Ca, P, O, Cu, and C elements. The core level spectrum of Ca 2p doublet (Fig. 7b) were assigned at 344.8 eV and 348.2 eV, corresponding to Ca 2p3/2 and Ca 2p1/2 respectively. The separation between these states was estimated to be 3.4 eV and was specified by Ca2+ oxidation state57–59. Further, a broad spectrum (Fig. 7c) of P 2p was deconvoluted into four characteristic peaks located at numerous binding energies of 130.11, 130.86, 131.52, and 132.27 eV caused by phosphate groups (PO43−) of TCP. The characteristic peaks at 130.11 eV and 130.86 eV for the electronic states 2p3/2 and 2p1/2 were derived by P-P bonding, and the remaining two peaks at 131.52 eV and 132.27 eV were illustrated by P-O-P of PO43− groups60–62 and were also confirmed by FTIR studies. Figure 7d revealed two components of the broad O1s peak obtained by the deconvolution method. A component of the low binding energy at 530.11 eV was assigned to O2− ions, while higher energy peak around 531.9 eV was attributed to adsorbed species of loosely bound O2 on the surface of materials63,64. The core level peak of Cu 2p (Fig. 7e) at binding energies 930.1 and 951.07 eV was ascribed by 2p3/2 and 2p1/2 respectively, which were the characteristic of Cu+. The peaks about 928.4, 935.2, and 940.7 eV were attributed to shake-up satellite peaks due to Cu2+ state65–70.
Fig. 7.
XPS spectra of the synthesized composites; (a) displaying survey of the composite THC8 and sintered at 1050 °C for 3 h. The core-level spectra of each elements (b) Ca 2P, (c) P 2P, (d) O 1s, (e) Cu 2p are revealing the different electronic states.
Physical and mechanical behavior
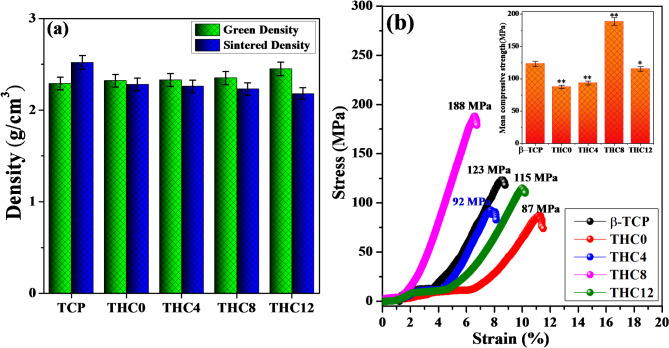
The physical parameters, including green and sintered densities, are illustrated in Fig. 8a; Table 2. These were calculated using mass and volume ratios of the prepared pellets. The green densities (density before sintered) of the β-TCP and four composites, THC0, THC4, THC8, and THC12, increased with increasing Cu nanoparticle content and lay within the range of 2.29 to 2.45 g/cm3, while the sintered densities decreased from 2.52 to 2.18 g/cm3. Because H3BO3 has been vaporized around 300 °C, and it might be completely removed at 1050 °C, leaving the voids within the composites that were clearly displayed in SEM images and confirmed by EDAX investigation.
Fig. 8.
Physical and mechanical behaviour of β-TCP and their composites (a) green and sintered density vs. composites, (b) stress vs. strain plot in compression mode with inset graph (*p < 0.05, **p < 0.01, one-way ANOVA with Tukey HSD test).
Table 2.
Physical and mechanical parameters including green and sintered density, Young’s modulus, compressive strength, break length, and fracture toughness of prepared pure β-TCP and composites.
| Sample’s name | Density | Young’s modulus (GPa) | Compressive strength (MPa) | Break length (mm) | Fracture toughness (MPa m1/2) | |
|---|---|---|---|---|---|---|
| Green (g/cm3) | Sintered (g/cm3) | |||||
| β-TCP | 2.29 ± 0.069 | 2.52 ± 0.076 | 1.42 ± 0.043 | 123 ± 3.69 | 0.92 ± 0.028 | 6.61 ± 0.20 |
| THC0 | 2.32 ± 0.070 | 2.28 ±0.068 | 0.77 ± 0.023 | 87 ± 2.61 | 0.91 ± 0.027 | 4.65 ± 0.14 |
| THC4 | 2.33 ± 0.070 | 2.26 ± 0.068 | 1.19 ± 0.036 | 92 ± 2.76 | 0.86 ± 0.026 | 4.82 ± 0.15 |
| THC8 | 2.35 ± 0.071 | 2.23 ±0.067 | 2.84 ± 0.085 | 188 ± 5.64 | 0.75 ± 0.023 | 9.12 ± 0.27 |
| THC12 | 2.45 ± 0.074 | 2.18 ± 0.065 | 1.15 ± 0.035 | 115 ± 3.45 | 0.83 ± 0.025 | 5.89 ± 0.18 |
Figure 8b revealed the stress vs. strain plot with an inset of a bar graph. The compressive strength of β-TCP was determined to be 123 MPa, and after adding H3BO3 (THC0), it was observed as 87 MPa and slightly improved up to 92 MPa with the substitution of Cu (4 wt%, THC4). Further, it was enhanced 53% higher than β-TCP and observed 188 MPa with increasing the doping concentration of Cu nanoparticles up to 8 wt% (THC8) due to well interconnected grains and overlapped hexagonal sheets of TCP. However, the strength of THC12 composites was further decreased due to flattened or irregular pores and poor interconnected large clusters of TCP, as clearly illustrated in SEM image. Compressive strength of all the composites was investigated by one-way ANOVA with a post hoc Tukey HSD test (*p < 0.05, **p < 0.01) and found to be significant. Fracture toughness and Young’s modulus of the prepared β-TCP and their composites varied from 4.82 to 9.12 MPa.m1/2 and 0.77 to 2.84 GPa respectively (Table 2 and Fig. S4). The composite THC8 exhibited maximum compressive strength, higher Young’s modulus, and excellent fracture toughness. These parameters can be varied according to the nature of the stress vs. strain curves.
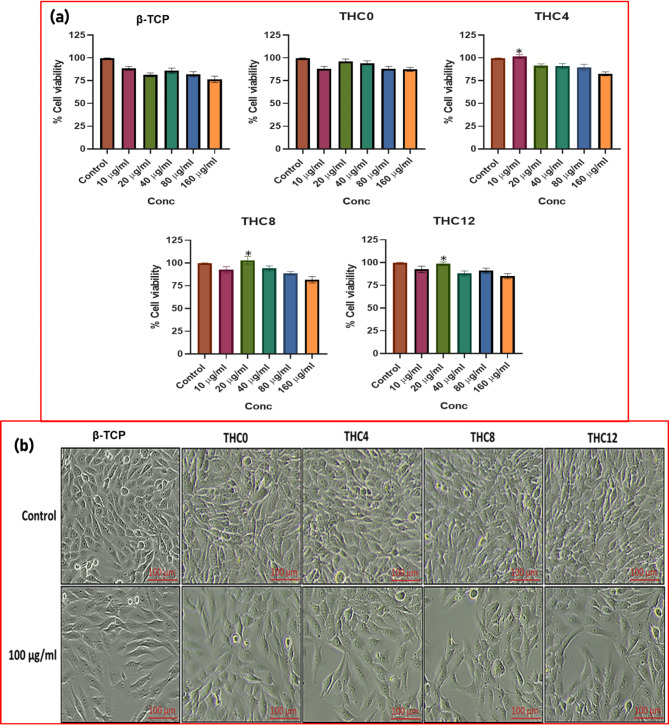
Cell viability of osteoblast cells
Cell viability is employed to investigate the effect of the composites in growth of MG-63 cells. The outcomes demonstrated that compared with control cells (untreated cells), osteoblast cells viability did not decrease as the amount of the doses increased. The results showed that at higher composite concentrations, there was no substantial reduction in cell growth (Fig. 9a). Morphological analysis of THC0, THC4, THC8, and THC12 exposed osteoblast cells demonstrated no or minimal cytotoxicity at higher concentrations (100 µg/ml) (Fig. 9b). Furthermore, β-TCP and their composites were incubated for 72 h, and it was found a more pronounced result at 20 µg/ml on increasing the concentration of Cu nanoparticles (Fig. 10a and b). Table S2 revealed the excellent mechanical properties and cell viability of β-TCP-H3BO3-Cu composite as compared to other existing alternatives71–75. Additionally, these composites were also performed on cervical cancer cell (SiHa) to investigate the anticancer activities (for more details see supplementary section).
Fig. 9.
(a) Percent cell viability of MG-63 cells treated with different concentrations of THC0, THC4, THC8 and THC12; (b) Morphological analysis of exposed MG-63 cells with THC0, THC4, THC8 and THC12 by Phase contrast microscopy.
Fig. 10.
(a) Cell viability assay of MG-63 cells showing the effect of TCP, THC0, THC4, THC8 and THC12 on different concentrations after 72 h treatment. *p < 0.05 **p < 0.01 ***p < 0.001. (b) The phase contrast microscopic images showing effect of THC0, THC4, THC8 and THC12 on higher concentrations after 72 h.
Antibacterial activity
Four composites (THC0, THC4, THC8, and THC12) were investigated to evaluate their antibacterial activity against pathogenic bacteria, including two strains of gram positive bacteria (B. subtilis and Streptococcus pyogene) and two strains of gram negative bacteria (S.typhi and E. coli) using disc diffusion process. We observed that all composites exhibited varying degrees of potential for preventing pathogens growth. The antibacterial activities of the composites are listed in Table 3; Fig. 11. The composite THC12 showed the highest antibacterial potency exclusively against B. subtilis. S. pyogene, S. typhi and E. coli. at 10 mg/ml, while composite THC0 showed less activity as compared to THC12, THC8 and THC4. Composites THC8 and THC4 displayed variable antimicrobial activities against the bacteria.
Table 3.
Antimicrobial susceptibility test of composites THC0, THC4, THC8, THC12 (10 mg/ml) against few pathogenic bacteria that causes skin and gastrointestinal diseases.
| Inhibition Zones (mm) | ||||
|---|---|---|---|---|
| Sample’s | Gram (+ve) pathogenic bacteria | Gram (-ve) pathogenic bacteria | ||
| Code | B. subtilis | S. pyogene | S. typhi | E. coli |
| THC0 | 10.16 ± 0.20 | 13.05 ± 0.51 | 11.02 ± 0.48 | 13.02 ± 0.39 |
| THC4 | 11.06 ± 0.21 | 13.03 ± 0.25 | 14.36 ± 0.43 | 14.83 ± 0.20 |
| THC8 | 13.06 ± 0.49 | 15.01 ± 0.25 | 15.56± 0.48 | 16.16 ± 0.37 |
| THC12 | 13.09 ± 0.80 | 15.04 ± 0.66 | 17.53 ± 0.71 | 16.09 ± 0.34 |
| Control positive | 16.06 ± 0.29 | 17.09 ± 0.49 | 18.04 ± 0.62 | 19.09 ± 0.39 |
Fig. 11.
Growth inhibition of bacterial strains caused by compounds (THC0, THC4, THC8, THC12) and C+, control positive (Gentamycin).
Antibacterial activity induced by copper
Copper serves as a crucial cofactor for various essential enzymes implicated in respiration and photosynthesis, including cytochrome C oxidase and ceruloplasmin, and its functions vary according to its oxidation state. The reduced Cu+ exhibits a preference for thiols and thioether groups, as observed in the side chains of cysteine and methionine, whereas the oxidized Cu2+ preferentially coordinates with oxygen or nitrogen groups, present in aspartate and glutamate, or the imidazole ring of histidine, respectively. Consequently, copper can assume numerous functions by interacting with diverse proteins, thereby playing a crucial part in various biological processes76. Copper nanoparticles (Cu-NPs) exhibit various antibacterial effects on bacteria, including adhesion to bacterial cell walls through electrostatic interactions. Cu-NPs, being positively charged ions, are attracted to negatively Antibacterial activity of copper-charged cell membranes, thereby compromising the integrity and functionality of the membrane and its associated proteins. The binding of copper to phospholipids may modify the physicochemical properties of the bacterial cell membrane, thereby reducing membrane fluidity and/or flexibility. Furthermore, this may elevate oxidative stress by increasing hydroxyl radicals at the membrane surface and may disrupt the electron transfer chain by direct or indirect contact with the quinone pool. Calvano et al.77 found that copper ions from a soluble copper salt induce significant membrane breakdown into lipids in E. coli. Numerous investigations examining the antibacterial efficacy of Cu-NPs and copper ions utilize both Gram-positive and Gram-negative bacteria to clarify the influence of their distinct cell wall structures on metal susceptibility and antibacterial effectiveness78. Gram-positive bacteria, including Bacillus subtilis and Staphylococcus aureus, possess a substantial quantity of amines and carboxyl groups on their cell surfaces, demonstrating a strong attraction for copper ions and copper-containing substances. Moreover, it has been established that the antibacterial efficacy of copper in Gram-negative bacteria mostly derives from its redox activity within the periplasmic region79. Rauf et al.80 investigated the impact of nanofibers composed of a copper(II)-based coordination polymer on E. coli and S. aureus. The study demonstrates markedly superior antibacterial efficacy and heightened membrane damage against E. coli in comparison to S. aureus, which is ascribed to the thinner cell wall of Gram-negative bacteria. Traditionally, it was believed that intracellular copper toxicity mostly arises from metal-mediated reactive oxygen species (ROS) production. Recent investigations have identified intracellular proteins as direct targets of copper toxicity. Elevated levels of copper are detrimental to prokaryotic cells, mainly owing to its redox characteristics. Numerous studies have associated the antibacterial efficacy of copper with its ability to oscillate between Cu+ and Cu2+, resulting in the generation of reactive oxygen species (ROS) in aerobic environments. The reaction necessitated a reducing agent (•O2−, NADPH oxidase from the respiratory chain, or intracellular thiols) to convert Cu2+ back to Cu1+, so completing the redox cycle and facilitating continued •OH generation. These free oxygen radicals may induce lipid peroxidation, resulting in diminished membrane fluidity and subsequent membrane rupture. Consequently, it is plausible that elevated cellular ROS levels are associated with the impairment of the bacterial cell membrane, thereby contributing to the antibacterial efficacy of copper78,81.
Minimum inhibitory concentrations (MIC’s) of the effective compounds
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the most effective compounds (THC12, THC8, and THC4) were assessed using the disc diffusion method to determine their bacteriostatic and bactericidal properties. The concentration effect of the active compounds is detailed in Table 4 and depicted in Fig. 12. The inhibitory effect of THC12 commenced at a concentration of 2.5 mg/ml, resulting in inhibition zones measuring 7.6 mm and 8.3 mm against B. subtilis and S. typhi, respectively. In contrast, compound THC4 demonstrated bacterial growth suppression for these strains at a concentration of 5 mg/ml, with inhibition zones of 7.7 mm and 9.3 mm, respectively.
Table 4.
MICs of the most effective compound against THC12, THC8 and THC4.
| Inhibition zones (mm) | |||
|---|---|---|---|
| Compound | Gram (+ve) pathogenic bacteria (B. subtilis) | Gram (-ve) pathogenic bacteria (S. typhi) | |
| THC12 | 1.25 | 00.00 ± 0.00 | 00.00 ± 0.00 |
| 2.50 | 07.6 ± 0.35 | 08.3 ± 0.26 | |
| 05.0 | 10.3 ± 0.32 | 11.6 ± 0.24 | |
| 10.0 | 13.8 ± 0.54 | 16.9 ± 0.14 | |
| 12.0 | 17.9 ± 0.35 | 19.3 ± 0.57 | |
| 15.0 | 23.4 ± 0.29 | 21.3 ± 0.48 | |
| 1.25 | 00.00 ± 0.00 | 00.00 ± 0.00 | |
| 2.50 | 00.00 ± 0.00 | 7.4 ± 0.26 | |
| 05.0 | 9.6 ± 0.31 | 10.5 ± 0.30 | |
| THC8 | 10.0 | 13.8 ± 0.20 | 15.2 ± 0.33 |
| 12.0 | 15.5 ± 0.28 | 16.2 ± 0.35 | |
| 15.0 | 18.7 ± 0.49 | 18.9 ± 0.12 | |
| THC4 | 1.25 | 00.00 ± 0.00 | 00.00 ± 0.00 |
| 2.50 | 00.00 ± 0.00 | 00.00 ± 0.00 | |
| 05.0 | 07.7 ± 0.56 | 9.3 ± 0.55 | |
| 10.0 | 10.8 ± 0.60 | 14.07 ± 0.46 | |
| 12.0 | 13.4 ± 0.89 | 15.8 ± 0.17 | |
| 15.0 | 15.2 ± 0.66 | 17.8 ± 0.49 | |
Fig. 12.
MICs of the effective compounds (THC12, THC8, THC4) against B. subtilis and S. typhi.
Minimum bactericidal concentrations (MBC’s) of the effective compounds
The MBC was established by the lack of bacterial growth in the tested strains, indicated by the inhibition zone that aligned with their lowest MICs. The THC12 and THC8 compounds demonstrated promising bactericidal activity against the examined pathogenic bacteria (B. subtilis and S. typhi), exhibiting a minimum bactericidal concentration (MBC) of 5 mg/ml, whereas the MBC for THC4 was found to be 10 mg/ml. The findings regarding the minimum inhibitory concentration and minimum bactericidal concentration of the effective compounds indicate that THC12, THC8, and THC4 are viable options for managing and preventing bacterial growth. THC12 demonstrated a strong ability to inhibit the growth of all bacterial strains tested, while THC8 and THC4 showed potential effectiveness against four bacterial strains.
Cytotoxicity study on drosophila
Trypan blue assay
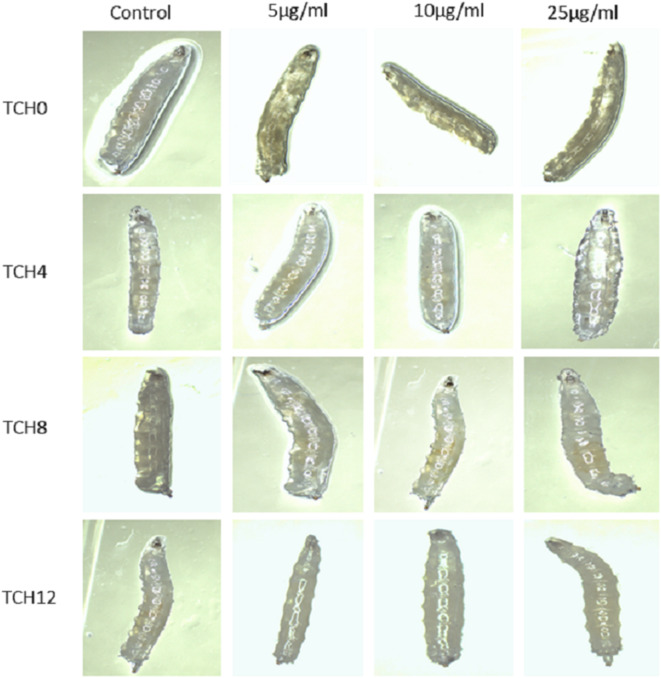
Trypan blue staining is useful for distinguishing between viable and dead cells. Nanoparticles such as hydroxyapatite (HAp NP), titania, and graphene oxide (GO) nanoparticles are well-known to induce damage to the larval gut, leading to the finding of trypan blue-positive stains within the larval gut82,83. However, some nanoparticles like curcumin, guar-gum, and platinum nanoparticles do not cause any damage to the gut84,85. Consistent with these results, the present study did not observe any trypan blue-positive staining across all concentrations of THC0, THC4, THC8, and THC12 (Fig. 13). This study advised that the existence of THC0, THC4, THC8, and THC12 does not harm the larval gut.
Fig. 13.
Showing Trypan blue negative results, in the presence of prepared composites THC0, THC4, THC8, and THC12 at different concentrations, which infers these composites do not harm the larval gut.
Evaluation of genotoxicity and cytotoxicity
DAPI staining was employed to detect nuclear fragmentation following exposure to THC0, THC4, THC8, and THC12 samples. Previous studies on nanoparticles (HApNP, Zirconia nanoparticles, and GO) have reported damaged nuclei by DAPI staining. However, nanoparticles like curcumin, guar-gum, and platinum were found not to cause damage to nuclei. In this investigation, nuclear fragmentation was seen at different concentrations (5 µg/ml, 10 µg/ml) of the composite THC0, THC4, THC8, and THC12 samples, while for (25 µg/ml) there were negligible nuclear fragments (Figs. 14 and 15). To assess the generation of oxidative stress following nanoparticle ingestion, larval guts were stained with DCFH-DA. Positive DCFH-DA staining has been reported in studies using HAp NP, Zr NP, and GO nanoparticles. Herein, we find less amount of ROS within the gut after the intake of different concentrations (2 µg/ml, 5 µg/ml, and 25 µg/ml) of THC0, THC4, THC8, and THC12 samples.
Fig. 14.
DAPI staining for THC0, THC4, THC8, and THC12 at different concentrations.
Fig. 15.
DCFH-DA staining for THC0, THC4, THC8, and THC12 at different concentrations.
Conclusions
In the present investigation, pure TCP was synthesised via a wet chemical precipitation route, and TCP based 3-D porous composites with H3BO3 and Cu nanoparticles were fabricated by the solid-state reaction method. Several characterization techniques were employed for the investigation of these fabricated composites, including XRD and FTIR for the phase and bonding mechanism, EDAX, XPS, and DLS spectroscopy for element compositions, electronic state, and average particle size of the composites. The porous morphology of the composites was revealed by SEM and TEM micrographs, and the TEM image also illustrates the hexagonal structure of TCP in the composite THC8. The mechanical characteristics of the THC8 composite were found to be excellent, including compressive strength (188 MPa), high Young’s modulus (2.84 GPa), and fracture toughness (9.11 MPa.m1/2) owing to the minimum break distance. The cell viability by MTT assay of osteoblast (MG-63) cells demonstrated no or minimal cytotoxicity at a higher concentration (100 µg/ml) and composites THC8 and THC12 are found to be significant for bone regeneration at 20 µg/ml after 72 h. As per the antimicrobial activity of the composites, THC12 was effective in inhibiting all pathogens. Despite this, genotoxicity and cytotoxicity tests were also performed on Drosophila melanogaster rearing. In accordance with these results, we did not observe any trypan blue-positive staining across all concentrations of the composites, which suggests that the existence of composites did not harm the larval gut. Therefore, the fabricated porous composites THC8 and THC12 are suitable for bone regeneration at the concentration of 20 µg/ml and will also protect against bacterial infections. Future research should prioritize in vivo studies to assess the long-term biocompatibility and efficacy of the TCP based composites for bone regeneration and cancer treatment. Additionally, investigating ways to enhance antimicrobial properties and optimize mechanical characteristics through material modifications will be beneficial. Exploring combination therapies and functionalizing the composites with bioactive molecules may further enhance therapeutic outcomes.
Method and materials
Several experiments were carried out to prepare materials with various characteristics. Whereas wet chemical and solid state reaction method have been used for the synthesis of β-TCP and its composites, cell culture and cell viability using MTT assays on different cells (MG-63, and Si-Ha) were utilized for the investigation of cell growth and anticancer properties. The antibacterial properties of the prepared composites were examined by employing the disk diffusion technique, and the cytotoxicity of the composites was studied on D. Melanogaster by DAPI and DCFH-DA techniques.
Synthesis of β-TCP
Calcium nitrate tetrahydrate (Ca(NO3)2·4H2O, ACS reagent, 99%, Sigma Aldrich, India) and di-sodium hydrogen phosphate dihydrate (Na2HPO4·2H2O, retaining purity, ≥ 99%, Merck, India) were utilized as starting materials for the synthesis of β-TCP via wet chemical method (Fig. 16), and sodium hydroxide (NaOH, Merck, India) was utilized to balance the pH during synthesis. Initially, 0.75 M (44.28 g) solution of Ca(NO3)2·4H2O into 250 mL of double distilled water (DDW) and 0.5 M (17.80 g) solution of Na2HPO4.2H2O into 200 mL of DDW were prepared separately in borosilicate glass beakers of 1000 mL and left at room temperature for half an hour. Further, the achieved solutions were mixed by magnetic stirrer, and then a few NaOH pallets were added to adjust the pH to 10, and was stirred at 85 °C for 3 h using a magnetic stirrer with a hot plate and allowed to cool at room temperature. The obtained milky white precipitate was washed several times using DDW, then dried in a hot oven at 150 °C for 1 h. Further, it was crushed into a fine powder using a mortar and pestle, and the calcination was carried out at 1100 °C for a duration of 3 h with a heating rate of 5 °C/m to achieve high purity and well-defined crystallinity. In this way, the obtained white powder of β-TCP was again crushed for 3 h and its formation was confirmed by XRD.
Fig. 16.
Schematic diagram of the synthesis of β-TCP by wet chemical method and its composites via solid state reaction method.
The solid state reaction technique was used to fabricate nanocomposites of β-TCP, boric acid (H3BO3, extra pure, minimum assay 99.5%, Himedia, India), and Cu nanopowder (Sigma Aldrich, Mumbai, India, purity > 99%) to investigate numerous properties, including physical, structural, mechanical, morphological, and biological. Four batches of six grams of the nanocomposite system (Table S3)  were separately stirred for 3 h in a glass beaker containing 50 mL of acetone. Furthermore, these wet batches were dried at 120 °C for 50 min, and then crushed by a mortar and pestle for 2 h. Thereafter, cylindrical pallets (10 mm diameter) were formed using a hydraulic press machine under an optimized pressure of 7 tons and sintered at 1050 °C for 3 h. The sintered pellets of four distinct composite samples i.e. 90TCP-10H3BO3 (THC0), 86TCP-10H3BO3-4Cu (THC4), 82TCP-10H3BO3-8Cu (THC8), and 78TCP-10H3BO3-12Cu (THC12) were successfully prepared. Furthermore, universal testing machine (UTM) was performed on the cross-sectional area of cylindrical pellets to investigate their mechanical properties, and then the deformed pellets were crushed into a fine powder for 6 h for further investigations such as XRD, DLS, SEM, TEM, XPS, and biological activities.
were separately stirred for 3 h in a glass beaker containing 50 mL of acetone. Furthermore, these wet batches were dried at 120 °C for 50 min, and then crushed by a mortar and pestle for 2 h. Thereafter, cylindrical pallets (10 mm diameter) were formed using a hydraulic press machine under an optimized pressure of 7 tons and sintered at 1050 °C for 3 h. The sintered pellets of four distinct composite samples i.e. 90TCP-10H3BO3 (THC0), 86TCP-10H3BO3-4Cu (THC4), 82TCP-10H3BO3-8Cu (THC8), and 78TCP-10H3BO3-12Cu (THC12) were successfully prepared. Furthermore, universal testing machine (UTM) was performed on the cross-sectional area of cylindrical pellets to investigate their mechanical properties, and then the deformed pellets were crushed into a fine powder for 6 h for further investigations such as XRD, DLS, SEM, TEM, XPS, and biological activities.
Cell culture
Dulbecco’s Modified Eagles Medium (DMEM) with 2 mM L-Glutamine and 10% Fetal Bovine Serum (FBS) was used to culture the human cervical cancer cell line (SiHa) and osteoblastic cell line (MG-63), purchased from the National Center for Cell Science (NCCS), Pune. The cells were incubated at 37 °C and 5% CO2 humidified in CO2 incubator. After 70 to 80% confluency of cells, the media was removed from the cells and trypsinized for further analysis. Trypan blue, 0.25% Trypsin-EDTA solution, FBS, Antibiotic-antimycotic (Ab/Am) solution, Dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide tetrazolium salt (MTT), and PBS: Phosphate Buffered Saline.
Cell viability by MTT assay
In 96-well plates, SiHa and MG-63 cells were seeded at 1 × 104 cells in each well. Cell viability of both untreated and treated cells was examined using a colorimetric reduction test with the tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye86,87. The SiHa and MG-63 cells were exposed to different concentrations of the composites THC0, THC4, THC8, and THC12 ranging from 50 to 300 µg/ml and 10 to 60 µg/ml, respectively, and then incubated for 24 h and 72 h at 37 °C with 5% CO2. Each well was added with 0.5 mg/mL of MTT (Himedia, Pennsylvania, USA) for 3 h at 37 °C. After aspirating the medium, 100 µL of DMSO has been added to each well to dissolve the formazan crystals. The proportion of live cells was estimated using a microplate reader (BioTek, Epoch 2, USA) reading the plate at 540 nm. The statistical analysis was carried out using GraphPad Prism. The following formula is employed to determine cell viability:
Antibacterial activities
The antimicrobial activity of the composites THC0, THC4, THC8, and THC12 under optimal conditions was tested against gram positive (Streptococcus Pyogene, Bacillus subtilis) as well as gram negative (Salmonella typhi, Pseudomonas aeruginosa and E. coli) bacteria. The strain was achieved from the culture collection of the Microbiology Department. Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow, India.
Inoculums preparation
All bacterial strains were subcultured for 24 h at 37 °C in Mueller-Hinton agar broth. A standardized bacterial suspension was produced by suspending a colony of bacteria in 10 mm of Mueller-Hinton agar broth medium using a sterilized wire loop. The dilutions have been employed to prepare the bacterial stock solutions for the disc diffusion testing.
Preparation of culture media
The liquid nutrition broth was utilized to inoculate the bacterial colonies, which were then cultured at 37 °C in the evening with 200 rpm of agitation. Subsequently, the McFarland half-turbidity criteria were applied to each broth culture to achieve approximately 1 × 108 CFU/mL88. Similarly, Mueller-Hinton medium was made in accordance with the instructions provided by manufacturer to assist as growth media in the agar disc diffusion test. 15 mL of prepared Mueller-Hinton agar medium were introduced into the culture plates. The agar plates were cooled for 2 h for them to solidify. The agar plates have been incubated for twenty-four hours at 37 °C to ensure sterility89.
Disc diffusion experiment to investigate antibacterial activity
A disc diffusion experiment was utilized to determine the antibacterial activity of each drug. An ultimate concentration of 10 mg/disc was achieved by separately dissolving 10 mg of the composites in 1 ml of DMSO and then transferring the mixture to an 8 mm-diameter sterile filter paper disc. Filter paper discs loaded with composites. Filter paper discs have been placed over solid sterile Mueller-Hinton Agar plates. As a positive control, gentamicin discs measuring 10 µg were also loaded. Finally, the plates have been incubated for twenty-four hours at 37 °C90. Following incubation, plates are examined, and the Vernier caliper method is employed to estimate the diameter of the inhibitory area. Bacterial cultures with an inhibitory area greater than 7 mm in diameter were considered that given composite is resistant to bacteria91.
Minimum inhibitory concentrations (MIC)
The minimum inhibitory concentration is characterized as the lowest level of the antimicrobial substance that prevents microbial proliferation following a 24-hour incubation period. The most effective compounds exhibiting strong antibacterial activity at 10 mg/ml were analyzed to determine their minimum inhibitory concentration using the disk diffusion method, and their efficacy in controlling bacterial strains was evaluated. Various concentrations of the active compounds (1.25, 2.5, 5.0, 10.0, 12.5, and 15.0 mg/ml) were meticulously prepared by dissolving 50 mg in 2.5 ml of ethanol, followed by sterilization using a Millipore filter. The appropriate amounts were then applied to sterilized filter paper discs (8 mm in diameter). Mueller-Hinton Agar was prepared in sterile Petri dishes and inoculated with suspensions of the infectious bacterial strains. The filter paper discs, infused with varying concentrations of the active compounds, were positioned atop the Mueller-Hinton agar plates. The plates were stored in the refrigerator at 4 °C for 2 h, followed by incubation at 35 °C for a duration of 24 h. The measurement of inhibition zones was conducted using a Vernier calliper, with results documented in relation to the concentrations of the active compound92.
Determination of minimum bactericidal concentrations (MBC’s)
Streaks were obtained from the two lowest concentrations of the compound plates showing no visible growth (based on the inhibition zone of MIC plates) and sub cultured onto sterile Muller Hinton agar (MHA) plates. The plates were incubated at 35 °C for 24 h and subsequently examined for bacterial growth in relation to the concentration of the compound. The minimum concentration of the compound was determined as the level at which no bacterial growth was observed on the freshly inoculated agar plates93.
Cytotoxicity test on drosophila
D. melanogaster rearing
Oregon-R flies were utilized in the present study. To prepare fly meal, a solution was made by mixing 0.4 g of Type-I agar-agar, 2 g of sucrose, 2.5 g of maize meal, and 1.25 g of yeast in 50 ml of milliQ water, as followed by Priyadarsini et al.94 This mixture was then supplemented with 150 µl of propionic acid and 250 µl of Nipagin under a laminar airflow. Propionic acid fosters larval growth and survival, whereas Nipagin has antimicrobial properties. Composite samples THC0, THC4, THC8, and THC12 were prepared at varying concentrations of 2 µg/ml, 5 µg/ml, and 25 µg/ml and introduced into fly meals. The ratio of male to female transferred flies was 3:5, and they were housed at 25 °C under 12 h light/dark cycles.
Trypan blue assay
To assess damage to gut epithelial cells, third-instar larvae were fed with composite nanoparticles THC0, THC4, THC8, and THC12 at (2 µg/ml, 5 µg/ml, and 25 µg/ml) concentrations. Six larvae from each concentration were taken and incubated with Trypan blue, a dye that stains dead cell membranes, as followed by Nayak et al.95 Place the larvae in a rocking shaker (ROCKYMAX) for 30 min. After incubation, the larvae were rinsed using phosphate-buffered saline (PBS) for 15 min to remove any excess stains. Subsequently, to assess any damage to the gut epithelial cells, the larvae were examined under a stereomicroscope (Motic-SMZ171).
Evaluation of genotoxicity and cytotoxicity
To investigate the effects of THC0, THC4, THC8, and THC12, we dissected the guts of the larvae that had been fed with these composites. Subsequently, the samples were stained with DAPI and DCFH-DA, as described by Priyadasini et al.96 DAPI binds to the AT region of DNA and stains the nucleus, while DCFH-DA was employed to detect mitochondrial ROS as a marker of cellular stress.
Sample characterizations
Powder XRD data were obtained using a Rigaku Ultima IV instrument, Japan within the two theta range of 10° to 70°. The scanning rate was fixed at 4° per minute with a step size of 0.02, utilizing Cu-Kα1 radiation (λ = 1.5405 Å) at an operational setting of 40 kV and 40 mA. In order to determine the crystallite sizes of both β-TCP and composites, Scherrer formula was used71.
 |
1 |
where D is crystallite size, 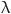 is the X-ray wavelength, β is FWHM of diffraction peaks, and θ is the Bragg angle. The crystallinity of the samples was determined based on the intensity of the peaks and the area under the diffraction peak. The crystallinity was measured by calculating the ratio of the area of crystalline peaks to total area, which includes both crystalline and amorphous peaks. The crystallinity (in percentage) was estimated using the following equation97.
is the X-ray wavelength, β is FWHM of diffraction peaks, and θ is the Bragg angle. The crystallinity of the samples was determined based on the intensity of the peaks and the area under the diffraction peak. The crystallinity was measured by calculating the ratio of the area of crystalline peaks to total area, which includes both crystalline and amorphous peaks. The crystallinity (in percentage) was estimated using the following equation97.
| 2 |
Further, Match Software was employed to determine the major and minor phases of the all composites. The crystal structures were created by VESTA software with CIF files collected from the Crystallographic Open Database (COD)98,99. Besides, FTIR spectroscopy (Perkin Elmer Spectrum 100 spectrophotometer) was utilized to study the vibrational modes within the wavenumber range of 400–4000 cm−1 at room temperature. X-ray Photo Spectroscopy (XPS) (Kα, Thermo Fisher Scientific) was used to study the binding energy and electronic transition state of the elements of a composite THC8, and it was performed on the pellet with 12 mm and 2 mm of diameter and thickness. UV-visible spectroscopy was conducted on composites using the ‘Thermo Scientific Evolution 201’ spectrometer, which operates within a fixed wavelength range of 250–800 nm. In addition, an investigation of the structural variations within the prepared samples was carried out by evaluating their optical properties using UV-Visible spectroscopy. Further, to determine the optical energy gap (Eg) of the samples, Tauc’s plots were generated and briefly analyzed using Eq. (3)100.
| 3 |
where α  represents the absorption coefficient, hν is the photon energy, and t is equal to 1/2, indicating indirect transition. The parameter Eg signifies the band gap energy of the samples. Additionally, the skin depth of the prepared composites characterizes the distance (in cm) an optical beam may penetrate into the synthesized composites before being scattered. Consequently, the skin depth (δ) is inversely proportional to α and is expressed by the following formula100.
represents the absorption coefficient, hν is the photon energy, and t is equal to 1/2, indicating indirect transition. The parameter Eg signifies the band gap energy of the samples. Additionally, the skin depth of the prepared composites characterizes the distance (in cm) an optical beam may penetrate into the synthesized composites before being scattered. Consequently, the skin depth (δ) is inversely proportional to α and is expressed by the following formula100.
| 4 |
DLS technique was used to measure the particle size by NZS90 zeta nanosizer (Malvern Instruments, UK) using the Stokes-Einstein equation71.
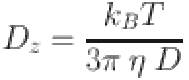 |
5 |
where Dz is the particle diameter, kB, T, η, and D denote the Boltzmann’s constant, temperature, viscosity, and diffusion coefficient, respectively. The morphological features of the prepared composites were observed using scanning electron microscopy (SEM, JSM-7610 F, JEOL, Japan). The prepared samples were coated on a copper stub by Pt-Pd using an auto-fine coater (JEOL, EC-32010CC; Japan), and the elemental composition was analyzed by EDAX (JSM-7610 F) on a captured SEM micrograph. SEM images were used to assess the grains morphology and size. The grain size was quantitatively analyzed by calculating the average diameter of the grains from the SEM images using ImageJ software. EDAX was attached to SEM for elemental analysis. Quantitative analysis was conducted by measuring the relative intensities of the characteristic X-ray peaks of the detected elements. The structural analyses of the composites were conducted using field emission transmission electron microscopy (FETEM, JEOL 2100 F, Japan) operated at 200 kV. A diluted suspension was prepared by dissolving 1 mg of the composite in 1 mL of toluene, followed by a 45-minute sonication for TEM measurements. The resulting suspension was applied to a carbon grid, allowed to dry for 40 min at 40 °C, and next vacuum-sealed for 12 h. Then, the desired TEM micrographs and selected area electron diffraction (SAED) patterns were taken.
In order to investigate the mechanical performances, a universal testing machine (UTM, Tinius Olsen H50KL, UK) was used on solid cylindrical pellets with 10 mm diameter and 15 mm height. Young’s modulus as well as fracture toughness were estimated by Eqs. (6) and (7) (stress - strain curves)101.
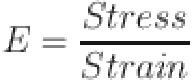 |
6 |
where E denotes Young’s modulus102,103.
| 7 |
where Kc, Y, σ, and α denote fracture toughness, geometrical constant (Y = 1), maximum stress, and break distance, respectively.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors are thankfully acknowledged to the Centre of Excellence scheme of Government of Uttar Pradesh for providing powder X-Ray Diffraction (PXRD) facility at Department of Physics and cell culture facility in Molecular and Human Genetics Laboratory, Department of Zoology, University of Lucknow, Lucknow. Authors also acknowledge to Research Grant Fund from DBT-BUILDER New Delhi, India.
Author contributions
S.K.A.: Conceptualization, Methodology, Writing- Original draft, Software. R.K.M.: Data curation, Writing- Original draft. Shweta: Formal analysis. S.K.: Visualization, Investigation. A.S.: Data curation, Formal analysis. S.P.: Methodology, Writing- Original draft. S.S.: Methodology, Writing- Original draft. S.K.: Formal analysis. Z.F.: Formal analysis. S.K.Y.: Resources. Monisha Banerjee: Writing - review & editing. M.M.: Writing - review & editing. N.M.: Resources. C.R.G.: Supervision, Writing - review & editing.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arbex, L. et al. Physio-mechanical and biological effects due to surface area modifications of 3D printed β-tri- calcium phosphate: An in vitro study. Ann. 3D Print. Med.8, 100078 (2022). [Google Scholar]
- 2.Bhardwaj, R., Singh, J., Kapila, R. & Boparai, R. S. Comparision of ilizarov ring fixator and rail fixator in infected nonunion of long bones: A retrospective followup study. Indian J. Orthop.53, 82–88 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mediero, A. et al. Ticagrelor regulates osteoblast and osteoclast function and promotes bone formation in vivo via an adenosine-dependent mechanism. FASEB J.30, 3887–3900 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolk, A. et al. Current trends and future perspectives of bone substitute materials—from space holders to innovative biomaterials. J. Cranio Maxillofac. Surg.40, 706–718 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Damien, C. J. & Parsons, J. R. Bone graft and bone graft substitutes: A review of current technology and applications. J. Appl. Biomater.2, 187–208 (1991). [DOI] [PubMed] [Google Scholar]
- 6.Trajkovski, B., Petersen, A., Strube, P., Mehta, M. & Duda, G. N. Intra-operatively customized implant coating strategies for local and controlled drug delivery to bone. Adv. Drug Deliv. Rev.64, 1142–1151 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Fadeeva, I. V. et al. Antibacterial and cell-friendly copper-substituted tricalcium phosphate ceramics for biomedical implant applications. Mater. Sci. Eng. C129, 112410 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Aoki, H. Science and Medical Applications of Hydroxyapatite (JAAS, 1991).
- 9.Rey, C. et al. 1.11 bioactive calcium phosphate compounds: Physical chemistry. Compr. Biomater. II1, 187–221 (2017). [Google Scholar]
- 10.Probst, F. A. et al. Bone regeneration of minipig mandibular defect by adipose derived mesenchymal stem cells seeded tri-calcium phosphate- poly(D,L-lactide-co-glycolide) scaffolds. Sci. Rep.10, 2062 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohner, M. & Santoni, B. L. G. Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater.113, 23–41 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Berger, G., Gildenhaar, R., Ploska, U., Driessens, F. C. M. & Planell, J. A. Short-term dissolution behaviour of some calcium phosphate cements and ceramics. J. Mater. Sci. Lett.16, 1267–1269 (1997). [Google Scholar]
- 13.Knabe, C. et al. Morphological evaluation of osteoblasts cultured on different calcium phosphate ceramics. Biomaterials18, 1339–1347 (1997). [DOI] [PubMed] [Google Scholar]
- 14.Fadeeva, I. V. et al. Copper-substituted tricalcium phosphates. Dokl. Chem.471, 384–387 (2016). [Google Scholar]
- 15.Dorozhkin, S. V. Bioceramics of calcium orthophosphates. Biomaterials31, 1465–1485 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Fielding, G. A., Bandyopadhyay, A. & Bose, S. Effects of silica and zinc oxide doping on mechanical and biological properties of 3D printed tricalcium phosphate tissue engineering scaffolds. Dent. Mater.28, 113–122 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Ghannam, A. Bone reconstruction: From bioceramics to tissue engineering. Expert Rev. Med. Devices2, 87–101 (2005). [DOI] [PubMed] [Google Scholar]
- 18.El-Ghannam, A. et al. A ceramic-based anticancer drug delivery system to treat breast cancer. J. Mater. Sci. Mater. Med.21, 2701–2710 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Cao, B., Yang, M., Zhu, Y., Qu, X. & Mao, C. Stem cells loaded with nanoparticles as a drug carrier for in vivo breast Cancer Therapy. Adv. Mater.26, 4627–4631 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selim, M. E. & Hendi, A. A. Gold nanoparticles induce apoptosis in MCF-7 human breast cancer cells. Asian Pac. J. Cancer Prev. APJCP13, 1617–1620 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Zhang, L. et al. Selective metabolic effects of gold nanorods on normal and cancer cells and their application in anticancer drug screening. Biomaterials34, 7117–7126 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Fiorillo, M. et al. Graphene oxide selectively targets cancer stem cells, across multiple tumor types: Implications for non-toxic cancer treatment, via ‘differentiation-based nano-therapy’. Oncotarget6, 3553–3562 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto, N., Sato, K., Yoshida, K., Hashimoto, K. & Toda, Y. Preparation and characterization of β-tricalcium phosphate co-doped with monovalent and divalent antibacterial metal ions. Acta Biomater.5, 3157–3164 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Liang, C. et al. Tumor metastasis inhibition by imaging-guided photothermal therapy with single-walled carbon nanotubes. Adv. Mater. Deerfield Beach Fla.26, 5646–5652 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Harb, S. V. et al. PLA-TCP-ZnO scaffolds for bone tissue engineering via 3D printing. https://inis.iaea.org/search/search.aspx?orig_q=RN:52111516 (2021).
- 26.Hu, X. et al. Fabrication of 3D gel-printed β-tricalcium phosphate/titanium dioxide porous scaffolds for cancellous bone tissue engineering. Int. J. Bioprinting9 (2023). [DOI] [PMC free article] [PubMed]
- 27.Amaral, S. S. et al. β-TCP/S53P4 scaffolds obtained by gel casting: Synthesis, properties, and biomedical applications. Bioengineering10, 597 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, X. et al. Zn-and Mg-containing tricalcium phosphates-based adjuvants for cancer immunotherapy. Sci. Rep.3, 2203 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.European Centre for Disease Prevention and Control. Surveillance of antimicrobial resistance in Europe 2018. Stockh. ECDC (2019).
- 30.Liu, L. et al. In vitro and in vivo mechanism of hepatocellular carcinoma inhibition by β-TCP nanoparticles. Int. J. Nanomed.14, 3491–3502 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharifipour, E. et al. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect. Dis.20, 1–7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Vidal, C. et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect.27, 83–88 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallardo-Williams, M. T., Maronpot, R. R., Wine, R. N., Brunssen, S. H. & Chapin, R. E. Inhibition of the enzymatic activity of prostate‐specific antigen by boric acid and 3‐nitrophenyl boronic acid. Prostate54, 44–49 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Barranco, W. T. & Eckhert, C. D. Boric acid inhibits human prostate cancer cell proliferation. Cancer Lett.216, 21–29 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Meacham, S. L., Elwell, K. E., Ziegler, S. & Carper, S. W. Boric acid inhibits cell growth in breast and prostate cancer cell lines. In Advances in Plant and Animal Boron Nutrition: Proceedings of the 3rd International Symposium on all Aspects of Plant and Animal Boron Nutrition 299–306 (Springer, 2007).
- 36.Gölge, U. H. et al. Effects of boric acid on fracture healing: An experimental study. Biol. Trace Elem. Res.167, 264–271 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Singh, A. K. et al. Effects of boric acid supplementation on bone health in crossbred calves under tropical condition. J. Trace Elem. Med. Biol.63, 126647 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Ulu, M. et al. Effects of boric acid on bone formation after maxillary sinus floor augmentation in rabbits. Oral Maxillofac. Surg.22, 443–450 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Adriztina, I., Adenin, L. I. & Lubis, Y. M. Efficacy of boric acid as a treatment of choice for chronic suppurative otitis media and its ototoxicity. Korean J. Fam. Med.39, 2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dinesh, S., Ajit, P., Madhav, P., Chetan, P. & Prachi, P. Study of antifungal activity of boric acid on vaginal pathogens. Int. J. Adv. Biotechnol. Res.4, 319–323 (2013). [Google Scholar]
- 41.Hołota, M. et al. In vitro anticancer properties of copper metallodendrimers. Biomolecules9, 155 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li, J. et al. Efficient label-free chemiluminescent immunosensor based on dual functional cupric oxide nanorods as peroxidase mimics. Biosens. Bioelectron.100, 304–311 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin, B. et al. Acidic pH and high-H2O2 dual tumor microenvironment-responsive nanocatalytic graphene oxide for cancer selective therapy and recognition. ACS Appl. Mater. Interfaces11, 11157–11166 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Glenske, K. et al. Applications of metals for bone regeneration. Int. J. Mol. Sci.19, 826 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, H. et al. Evaluation of borate bioactive glass scaffolds as a controlled delivery system for copper ions in stimulating osteogenesis and angiogenesis in bone healing. J. Mater. Chem. B. 2, 8547–8557 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Dickens, B., Schroeder, L. & Brown, W. Crystallographic studies of the role of mg as a stabilizing impurity in β-Ca3(PO4)2. The crystal structure of pure β-Ca3(PO4)2. J. Solid State Chem.10, 232–248 (1974). [Google Scholar]
- 47.Matsunaga, K., Kubota, T., Toyoura, K. & Nakamura, A. First-principles calculations of divalent substitution of Ca2+ in tricalcium phosphates. Acta Biomater.23, 329–337 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Jay, E. et al. Predicted energies and structures of β-Ca3(PO4)2. J. Solid State Chem.183, 2261–2267 (2010). [Google Scholar]
- 49.Corno, M., Busco, C., Civalleri, B. & Ugliengo, P. Periodic ab initio study of structural and vibrational features of hexagonal hydroxyapatite Ca10(PO4)6(OH)2. Phys. Chem. Chem. Phys. PCCP8, 2464–2472 (2006). [DOI] [PubMed] [Google Scholar]
- 50.De Filho, S., Serra, O. A. & P. C. & Reverse Microemulsion synthesis, structure, and luminescence of nanosized REPO4:ln 3+ (RE = La, Y, Gd, or Yb, and ln = eu, tm, or Er). J. Phys. Chem. C115, 636–646 (2011). [Google Scholar]
- 51.Karmakar, B. Fundamentals of glass and glass nanocomposites. In Glass Nanocomposites 3–53 (Elsevier, 2016). 10.1016/B978-0-323-39309-6.00001-8.
- 52.Rair, D. et al. Synthesis and study by FTIR, 31P NMR and electrochemical impedance spectroscopy of vanadium zinc phosphate glasses prepared by sol–gel route. J. Non Cryst Solids432, 459–465 (2016). [Google Scholar]
- 53.Jobson, E., Baiker, A. & Wokaun, A. Adsorption of ammonia and methylamines on alumina and copper/alumina studied by dynamic Fourier-transform infrared experiments. J. Chem. Soc. Faraday Trans.86, 1131–1137 (1990). [Google Scholar]
- 54.Das, V., Tripathi, A. M., Borah, M. J., Dunford, N. T. & Deka, D. Cobalt-doped CaO catalyst synthesized and applied for algal biodiesel production. Renew. Energy161, 1110–1119 (2020). [Google Scholar]
- 55.Hadjiivanov, K. I. et al. Power of infrared and Raman spectroscopies to characterize metal-organic frameworks and investigate their interaction with guest molecules. Chem. Rev.121, 1286–1424 (2021). [DOI] [PubMed] [Google Scholar]
- 56.Obada, D., Dauda, E., Abifarin, J., Dodoo-Arhin, D. & Bansod, N. Mechanical properties of natural hydroxyapatite using low cold compaction pressure: Effect of sintering temperature. Mater. Chem. Phys.239, 122099 (2020). [Google Scholar]
- 57.Lee, C., Pode, R., Han, J. & Moon, D. Red electrophosphorescent top emission organic light-emitting device with Ca/ ag semitransparent cathode. Appl. Phys. Lett.89 (2006).
- 58.Sabbarwal, S., Dubey, A. K., Pandey, M. & Kumar, M. Synthesis of biocompatible, BSA capped fluorescent CaCO3 pre-nucleation nanoclusters for cell imaging applications. J. Mater. Chem. B8, 5729–5744 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Alagarsamy, S., Vincent John, A. J., Chen, S. M., Mariappan, K. & Sakthinathan, S. Tetrahedrally structured CaMoO4 nanospheres on heteroatom-doped carbon nanotubes for sensitive food additive electrochemical sensors. ACS Appl. Nano Mater.10.1021/acsanm.3c06056 (2024). [Google Scholar]
- 60.Xie, L. et al. Fe/Zn-modified tricalcium phosphate (TCP) biomaterials: Preparation and biological properties. RSC Adv.9, 781–789 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tian, B. et al. Supported black phosphorus nanosheets as hydrogen-evolving photocatalyst achieving 5.4% energy conversion efficiency at 353 K. Nat. Commun.9, 1397 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Hou, Y. et al. 2D black arsenic phosphorus and its application for anodes of lithium ion batteries. CrystEngComm22, 8228–8235 (2020). [Google Scholar]
- 63.Ievtushenko, A. et al. X-ray photoelectron spectroscopy study of highly-doped ZnO: Al, N films grown at O-rich conditions. J. Alloys Compd.722, 683–689 (2017). [Google Scholar]
- 64.Yoon, K. H., Choi, J. W. & Lee, D. H. Characteristics of ZnO thin films deposited onto Al/Si substrates by rf magnetron sputtering. Thin Solid Films302, 116–121 (1997). [Google Scholar]
- 65.Cooper, J. K. et al. CuBi2O4: Electronic structure, optical properties, and photoelectrochemical performance limitations of the photocathode. Chem. Mater.33, 934–945 (2021). [Google Scholar]
- 66.Kou, Y. et al. Synthesis of hollow Cu@Cu3−xP core-shell nanostructure as dual-functional catalyst with copper vacancy for enhancing chemical reduction and photocatalytic performance. Appl. Surf. Sci.589, 153031 (2022). [Google Scholar]
- 67.Yin, M. et al. Copper oxide nanocrystals. J. Am. Chem. Soc.127, 9506–9511 (2005). [DOI] [PubMed] [Google Scholar]
- 68.Sahai, A., Goswami, N., Kaushik, S. & Tripathi, S. Cu/Cu2O/CuO nanoparticles: Novel synthesis by exploding wire technique and extensive characterization. Appl. Surf. Sci.390, 974–983 (2016). [Google Scholar]
- 69.Yang, J. et al. Tuning the cu + species of Cu-based catalysts for direct synthesis of ethanol from syngas. New. J. Chem.45, 20832–20839 (2021). [Google Scholar]
- 70.Bagheri, M., Melillo, A., Ferrer, B., Masoomi, M. Y. & Garcia, H. Quasi-HKUST prepared via postsynthetic defect engineering for highly improved catalytic conversion of 4-nitrophenol. ACS Appl. Mater. Interfaces. 14, 978–989 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Avinashi, S. K. et al. Morphological, mechanical, and biological evolution of pure hydroxyapatite and its composites with titanium carbide for biomedical applications. Ceram. Int.48, 18475–18489 (2022). [Google Scholar]
- 72.Gautam, C. R., Kumar, S., Mishra, V. K. & Biradar, S. Synthesis, structural and 3-D architecture of lanthanum oxide added hydroxyapatite composites for bone implant applications: Enhanced microstructural and mechanical properties. Ceram. Int.43, 14114–14121 (2017). [Google Scholar]
- 73.Gautam, A. et al. Enhanced mechanical properties of hBN–ZrO2 composites and their biological activities on Drosophila melanogaster: Synthesis and characterization. RSC Adv.9, 40977–40996 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang, Z. et al. Fabrication, in vitro and in vivo properties of β-TCP/Zn composites. J. Alloys Compd.913, 165223 (2022). [Google Scholar]
- 75.Gautam, A. et al. Synthesis, structural, mechanical, and biological properties of HAp-ZrO2-hBN biocomposites for bone regeneration applications. Ceram. Int.47, 30203–30220 (2021). [Google Scholar]
- 76.Festa, R. A. & Thiele, D. J. Copper: An essential metal in biology. Curr. Biol.21, R877–R883 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Calvano, C. D. et al. MALDI-TOF mass spectrometry analysis of proteins and lipids in Escherichia coli exposed to copper ions and nanoparticles. J. Mass. Spectrom.51, 828–840 (2016). [DOI] [PubMed] [Google Scholar]
- 78.Wu, Y., Wu, W., Zhao, W. & Lan, X. Revealing the antibacterial mechanism of copper surfaces with controllable microstructures. Surf. Coat. Technol.395, 125911 (2020). [Google Scholar]
- 79.Macomber, L., Rensing, C. & Imlay, J. A. Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J. Bacteriol.189, 1616–1626 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rauf, A. et al. Copper (ii)-based coordination polymer nanofibers as a highly effective antibacterial material with a synergistic mechanism. Dalton Trans.48, 17810–17817 (2019). [DOI] [PubMed] [Google Scholar]
- 81.Dalecki, A. G., Crawford, C. L. & Wolschendorf, F. Copper and antibiotics: Discovery, modes of action, and opportunities for medicinal applications. Adv. Microb. Physiol.70, 193–260 (2017). [DOI] [PubMed] [Google Scholar]
- 82.Pappus, S. A. et al. A toxicity assessment of hydroxyapatite nanoparticles on development and behaviour of Drosophila melanogaster. J. Nanoparticle Res.19, 1–16 (2017). [Google Scholar]
- 83.Ekka, B. et al. Removal of cr (VI) by silica-titania core-shell nanocomposites: in vivo toxicity assessment of the adsorbent by Drosophila melanogaster. Ceram. Int.47, 19079–19089 (2021). [Google Scholar]
- 84.Mukherjee, S., Rananaware, P., Brahmkhatri, V. & Mishra, M. Polyvinylpyrrolidone-curcumin nanoconjugate as a biocompatible, non-toxic material for biological applications. J. Clust Sci.34, 395–414 (2023). [Google Scholar]
- 85.Bag, J. et al. Fe3O4 coated guargum nanoparticles as non-genotoxic materials for biological application. Int. J. Biol. Macromol.165, 333–345 (2020). [DOI] [PubMed] [Google Scholar]
- 86.Parveen, S. et al. Enhanced therapeutic efficacy of Piperlongumine for cancer treatment using nano-liposomes mediated delivery. Int. J. Pharm.643, 123212 (2023). [DOI] [PubMed] [Google Scholar]
- 87.Kumar, S., Parveen, S., Swaroop, S. & Banerjee, M. TNF-α and MMPs mediated mucus hypersecretion induced by cigarette smoke: An in vitro study. Toxicol. Vitro92, 105654 (2023). [DOI] [PubMed] [Google Scholar]
- 88.Kothari, S., Mishra, V., Bharat, S. & Tonpay, S. D. Antimicrobial activity and phytochemical screening of serial extracts from leaves of Aegle marmelos (Linn). Acta Pol. Pharm.68, 687–692 (2011). [PubMed] [Google Scholar]
- 89.Chaddha, A. S., Sharma, A., Singh, N. K., Shamsad, A. & Banerjee, M. Biotic-abiotic mingle in rock varnish formation: A new perspective. Chem. Geol.648, 121961 (2024). [Google Scholar]
- 90.Silva, A. P. et al. Antimicrobial activity and phytochemical analysis of organic extracts from cleome spinosa Jaqc. Front. Microbiol.7, 178975 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ganesan, P. et al. Antimicrobial activity of some actinomycetes from western ghats of Tamil Nadu, India. Alex. J. Med.53, 101–110 (2017). [Google Scholar]
- 92.Yilmaz, M. T. Minimum inhibitory and minimum bactericidal concentrations of boron compounds against several bacterial strains. Turk. J. Med. Sci.42, 1423–1429 (2012). [Google Scholar]
- 93.Mostafa, A. A. et al. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci.25, 361–366 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Priyadarsini, S., Mukherjee, S. & Mishra, M. Formulation of Drosophila food for various feeding studies. In Fundamental Approaches to Screen Abnormalities in Drosophila 1–13. 10.1007/978-1-4939-9756-5_1 (2020).
- 95.Nayak, P. et al. Coumarin-based noncytotoxicity fluorescent dye for tracking actin protein in in-vivo imaging. Chem. Res. Toxicol.36, 926–933 (2023). [DOI] [PubMed] [Google Scholar]
- 96.Priyadarsini, S., Mukherjee, S. & Mishra, M. Methodology to detect the abnormality of drosophila gut by various staining techniques. In Fundamental Approaches to Screen Abnormalities in Drosophila 51–64. 10.1007/978-1-4939-9756-5_5 (2020).
- 97.Kumari, S. et al. Structural, mechanical and biological properties of PMMA-ZrO2 nanocomposites for denture applications. Mater. Chem. Phys.295, 127089 (2023). [Google Scholar]
- 98.Vaitkus, A., Merkys, A. & Gražulis, S. Validation of the crystallography open database using the crystallographic information framework. J. Appl. Crystallogr.54, 661–672 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Momma, K. & Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr.44, 1272–1276 (2011). [Google Scholar]
- 100.Madheshiya, A. et al. Synthesis, physical, optical and structural properties of SrTiO3 borosilicate glasses with addition of CrO3. Bull. Mater. Sci.46, 34 (2023). [Google Scholar]
- 101.Shweta et al. Synergetic effects of boron nitride with waste zirconia: Evaluation of instantaneous fingerprint detection and mechanical properties for biomedical applications. J. Mech. Behav. Biomed. Mater.145, 106032 (2023). [DOI] [PubMed] [Google Scholar]
- 102.Shweta et al. Structural, morphological and mechanical insights from La2O3 doped machinable silicate glass ceramics for biomedical applications. Ceram. Int.49, 8801–8819 (2023). [Google Scholar]
- 103.Avinashi, S. K. et al. Fabrication of Novel 3-D nanocomposites of HAp–TiC–h-BN–ZrO2: enhanced mechanical performances and in vivo toxicity study for biomedical applications. ACS Biomater. Sci. Eng.. 10.1021/acsbiomaterials.3c01478 (2024). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.