Figure 4.
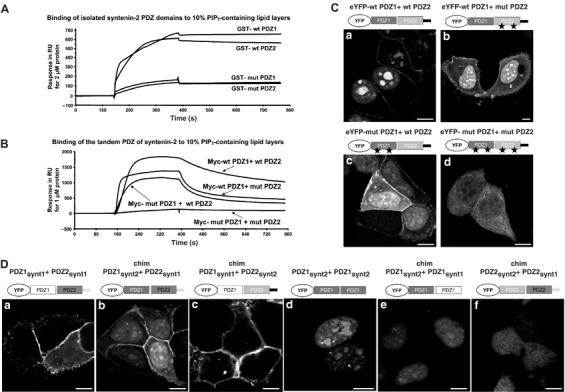
Respective contribution of syntenin-2 PDZ domains in PIP2 binding and targeting to specific subcellular PIP2 pools. (A) SPR sensograms showing the binding of the individual PDZ domains of syntenin-2 to 10% PIP2-containing lipid layers. Wild-type (wt) and mutant (mut) PDZ domains were purified as GST fusion proteins and perfused at 2 μM. Mutant PDZ domains (mut PDZ1 and mut PDZ2) were made by changing two lysine residues into alanine residues. (B) SPR sensograms showing the interaction of the tandem PDZ of syntenin-2 with 10% PIP2-containing lipid layers. Wild-type and mutant PDZ domains were purified as Myc fusion proteins and perfused at 1 μM over the sensorchip. (C) Subcellular localization of wild-type and mutant tandem PDZ of syntenin-2 in MCF-7 cells was analyzed by confocal microscopy. Proteins were expressed as eYFP fusions. (D) Confocal micrographs of MCF-7 cells expressing different combinations of the PDZ domains of syntenin-1 and syntenin-2 as indicated. Size bars are 10 μm.
