Figure 1.
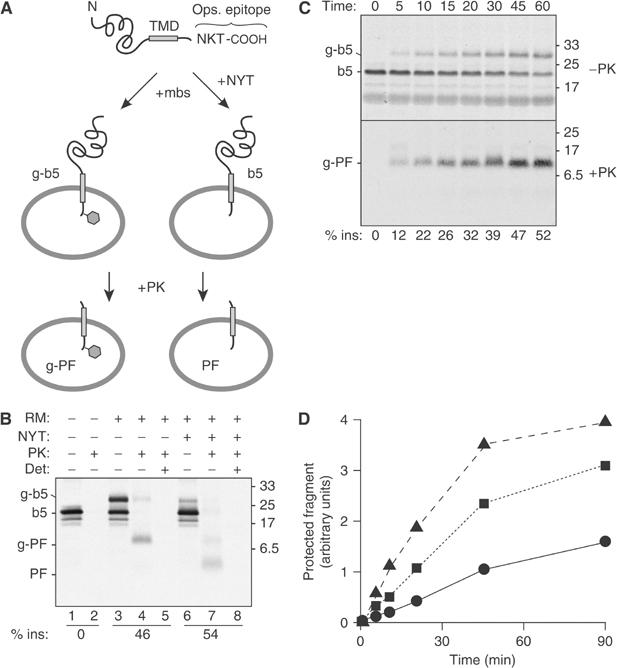
Protease protection assay for transmembrane insertion of a TA protein. (A) Illustration of the model TA protein construct (b5-Nglyc) and assay design. (B) In vitro translated b5-Nglyc was post-translationally incubated with ER-derived RMs in the absence or presence of a tripeptide inhibitor of glycosylation (NYT). The samples were then divided for digestion with PK in the presence or absence of the detergent TX100. Equal amounts of each sample were subsequently immunoprecipitated with antibodies against the C-terminal opsin tag prior to analysis by SDS–PAGE and autoradiography. The positions of the primary translation product (b5), glycosylated b5 (g-b5), and their respective protease-protected fragments (PF and g-PF) are indicated to the left. The efficiency of insertion (% ins) is indicated below the respective lanes. (C) Time course of glycosylation and translocation of b5-Nglyc. Translated b5-Nglyc was incubated with RMs for the indicated times. For each time point, total nondigested and PK-digested immunoprecipitated samples are shown in the upper and lower panel, respectively. A nonglycosylated PF band could not be detected even on longer exposures of the autoradiograph. (D) Time course of generation of b5-Nglyc PF with different loads of pig RM: 0.1 eq/μl (•); 0.35 eq/μl (▪); 0.7 eq/μl (▴). In all figures, numbers on the side of the panels indicate the position and size (in kDa) of Mr markers.
