Figure 2.
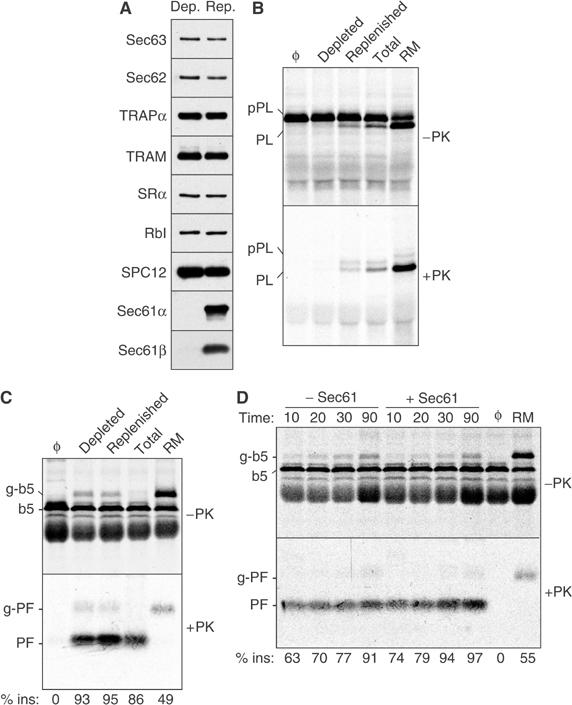
Transmembrane integration of b5-Nglyc is unaffected by Sec61 depletion. (A) Proteoliposomes reconstituted from a Sec61-depleted extract or a depleted extract replenished with purified Sec61 complex were analysed by immunoblotting against various translocation-related ER proteins (SRα, α subunit of the SRP receptor; RbI, ribophorin I; TRAM, translocating chain-association membrane protein; TRAPα, α subunit of the translocon-associated protein; SPC, signal peptidase complex). (B) The Sec61-depleted and replenished proteoliposomes from panel A were assayed for cotranslational translocation of pPL. Equal aliquots of the translocation reaction were either left untreated (top panel) or digested with PK (bottom panel) prior to analysis by SDS–PAGE. Translocation reactions containing native RM, proteoliposomes made with a total RM extract (Total), or without added vesicles (Φ) are also included for comparison. The positions of precursor (pPL) and signal-cleaved PL are indicated. (C) The various membranes analysed in panel B were assayed for b5-Nglyc translocation using the protease protection assay. (D) The time course of b5-Nglyc translocation into Sec61-depleted and replenished proteoliposomes was assayed by protease protection. Reactions lacking vesicles (Φ) or containing RM are also shown.
