Abstract
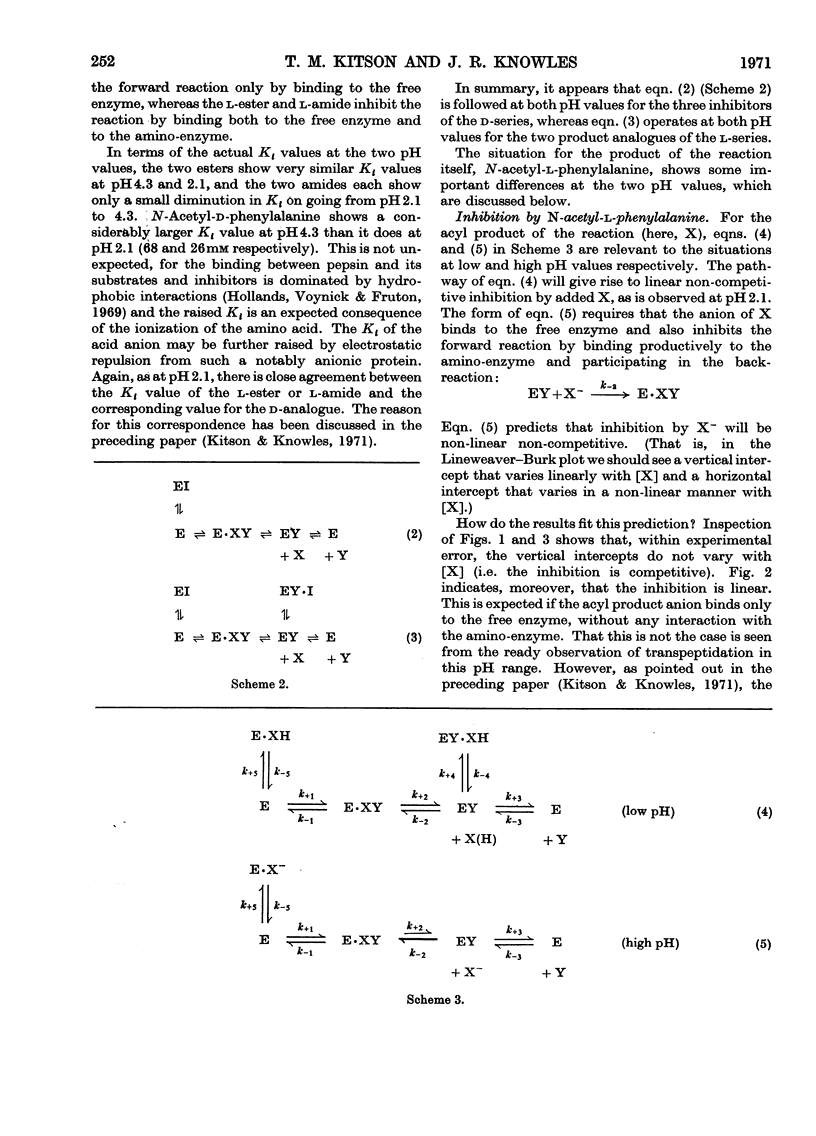
1. The inhibition of pepsin-catalysed hydrolysis of N-acetyl-l-phenylalanyl-l-phenylalanylglycine by the acyl product and product analogues was studied at pH4.3. 2. The acyl product, N-acetyl-l-phenylalanine, gives rise to linear competitive inhibition at pH4.3, whereas at pH2.1 it shows linear non-competitive behaviour. 3. The extent of transpeptidation to N-acetyl-l-[3H]phenylalanine during the pepsin-catalysed hydrolysis of N-acetyl-l-phenylalanyl-l-phenylalanyl-glycine is significant at pH4.7, but is undetectable at pH1.3. 4. Both the inhibition and transpeptidation experiments are consistent with the anion of the acceptor molecule being the substrate in pepsin-catalysed transpeptidation. This conclusion supports the formulation of pepsin-catalysed reactions put forward by Knowles et al. (1970).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAPLOW M., JENCKS W. P. THE CHYMOTRYPSIN-CATALYZED HYDROLYSIS AND SYNTHESIS OF N-ACETYL-L-TYROSINE HYDROXAMIC ACID. J Biol Chem. 1964 May;239:1640–1652. [PubMed] [Google Scholar]
- Cornish-Bowden A. J., Knowles J. R. The pH-dependence of pepsin-catalysed reactions. Biochem J. 1969 Jun;113(2):353–362. doi: 10.1042/bj1130353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRUTON J. S., FUJII S., KNAPPENBERGER M. H. The mechanism of pepsin action. Proc Natl Acad Sci U S A. 1961 Jun 15;47:759–761. doi: 10.1073/pnas.47.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
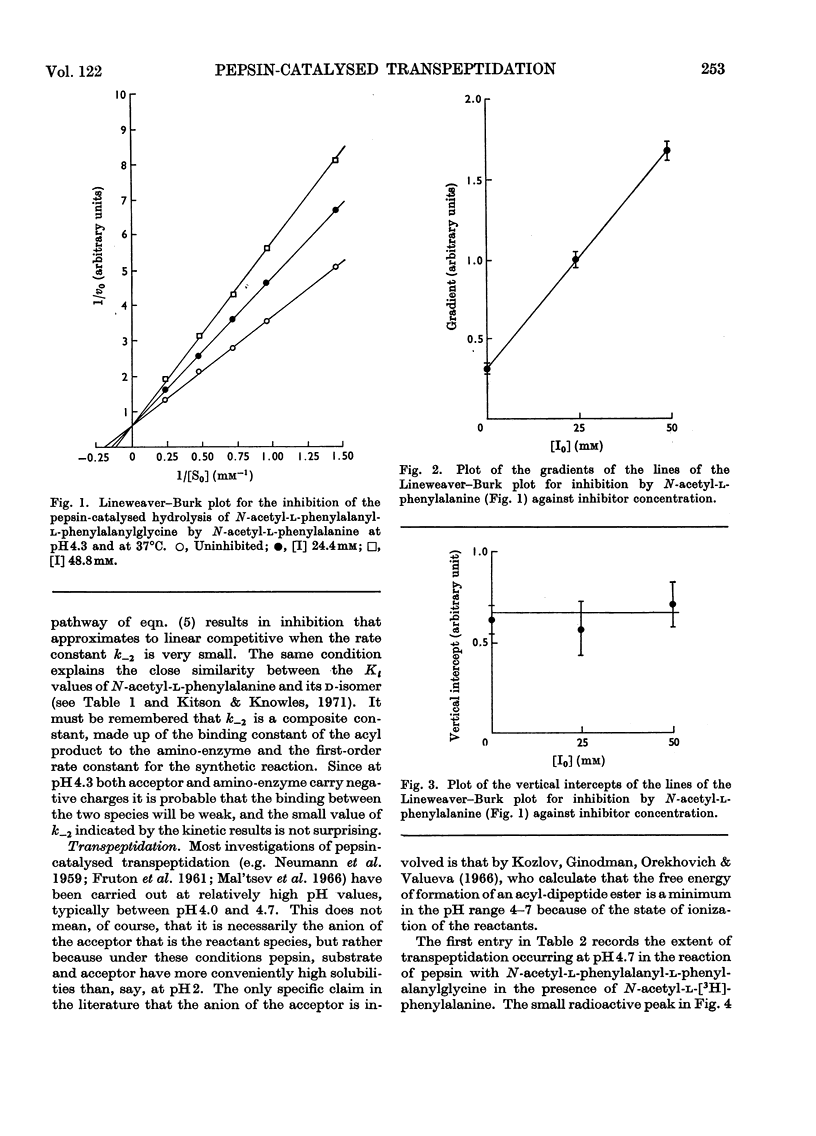
- Greenwell P., Knowles J. R., Sharp H. The inhibition of pepsin-catalysed reactions by products and product analogues. Kinetic evidence for ordered release of products. Biochem J. 1969 Jun;113(2):363–368. doi: 10.1042/bj1130363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu R. Y., Cleland W. W., Anderson L. Mechanism of action of the nonspecific phosphomonoesterase from potatoes. Biochemistry. 1966 Feb;5(2):799–807. doi: 10.1021/bi00866a055. [DOI] [PubMed] [Google Scholar]
- Kitson T. M., Knowles J. R. The inhibition of pepsin-catalysed reactions by structural and stereochemical product analogues. Biochem J. 1971 Apr;122(2):241–247. doi: 10.1042/bj1220241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles J. R. On the mechanism of action of pepsin. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):135–146. doi: 10.1098/rstb.1970.0016. [DOI] [PubMed] [Google Scholar]
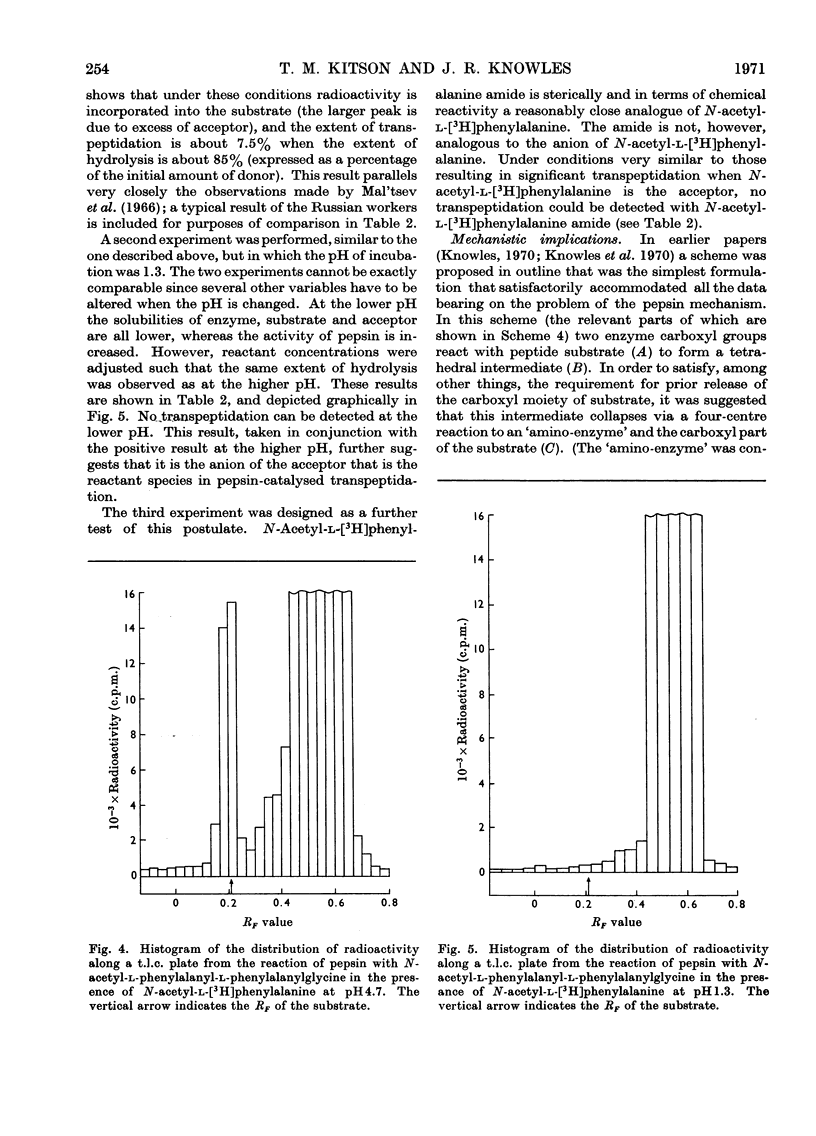
- Kozlov L. V., Ginodman L. M., Orekhovich V. N., Valueva T. A. Svobodnaia énergiia gidroliza peptidnoi sviazi i fermentativnyi sintez éfirov N-atsetildipeptidov. Biokhimiia. 1966 Mar-Apr;31(2):315–321. [PubMed] [Google Scholar]
- Mal'tsev N. I., Ginodman L. M., Orekhovich V. N., Valueva T. A., Akimova L. N. Issledovanie spetsifichnosti pepsina v reaktsiiakh transpeptidatsii. Biokhimiia. 1966 Sep-Oct;31(5):983–987. [PubMed] [Google Scholar]
- NEUMANN H., LEVIN Y., BERGER A., KATCHALSKI E. Pepsincatalysed transpeptidation of the amino-transfer type. Biochem J. 1959 Sep;73:33–41. doi: 10.1042/bj0730033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M. S., Stoddard M., Stein T. P. On the mechanism of the pepsin-catalyzed exchange of carboxylic acids with water-18O. J Am Chem Soc. 1970 May 6;92(9):2883–2890. doi: 10.1021/ja00712a047. [DOI] [PubMed] [Google Scholar]


