Abstract
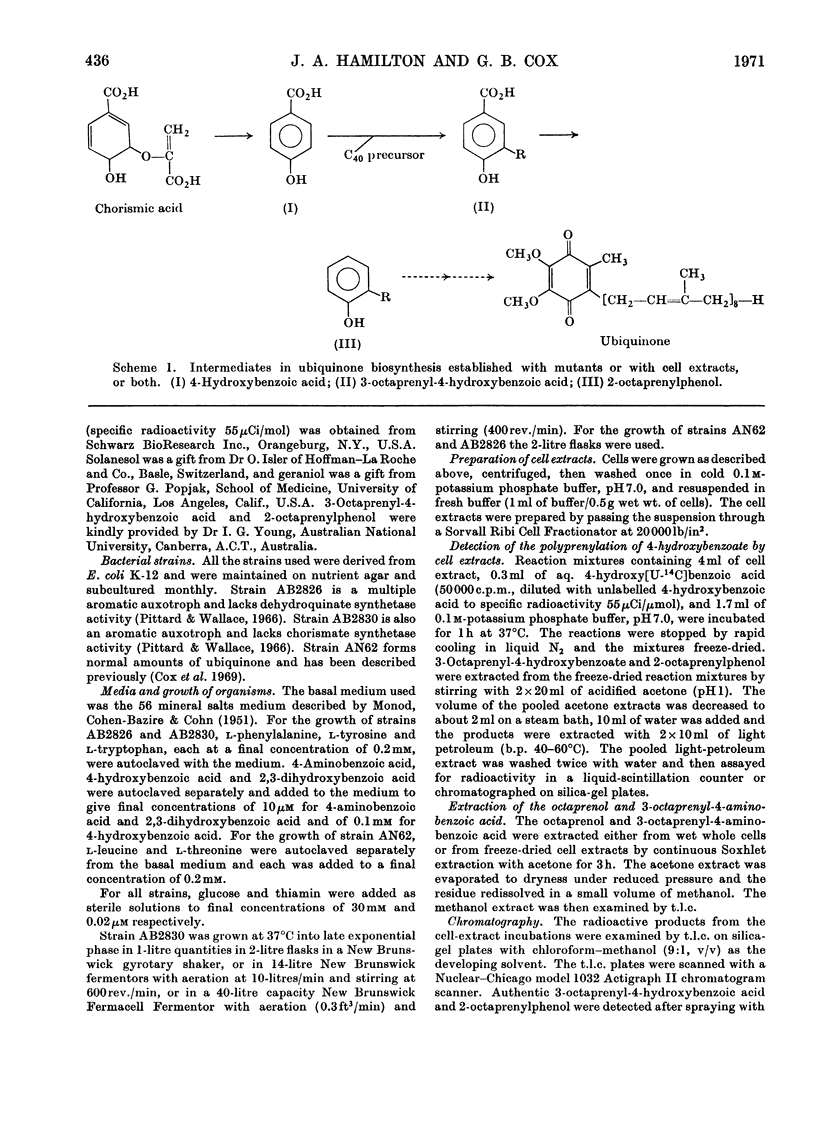
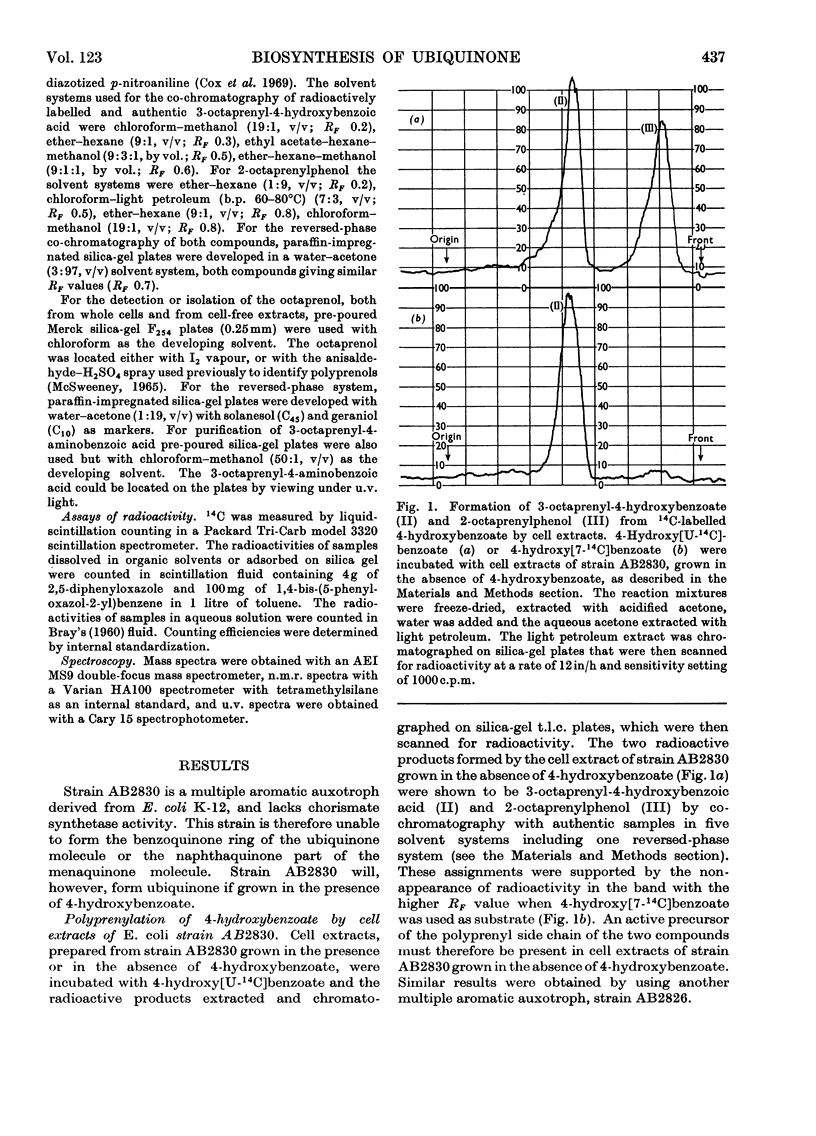
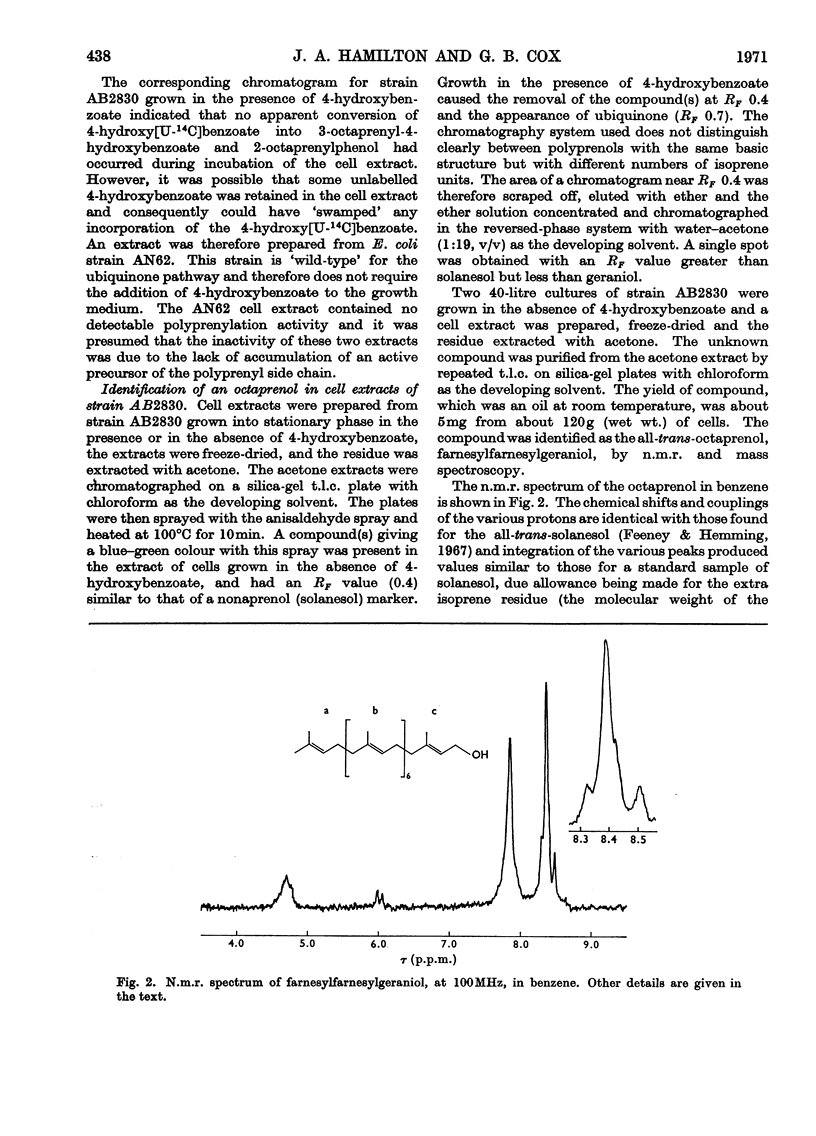
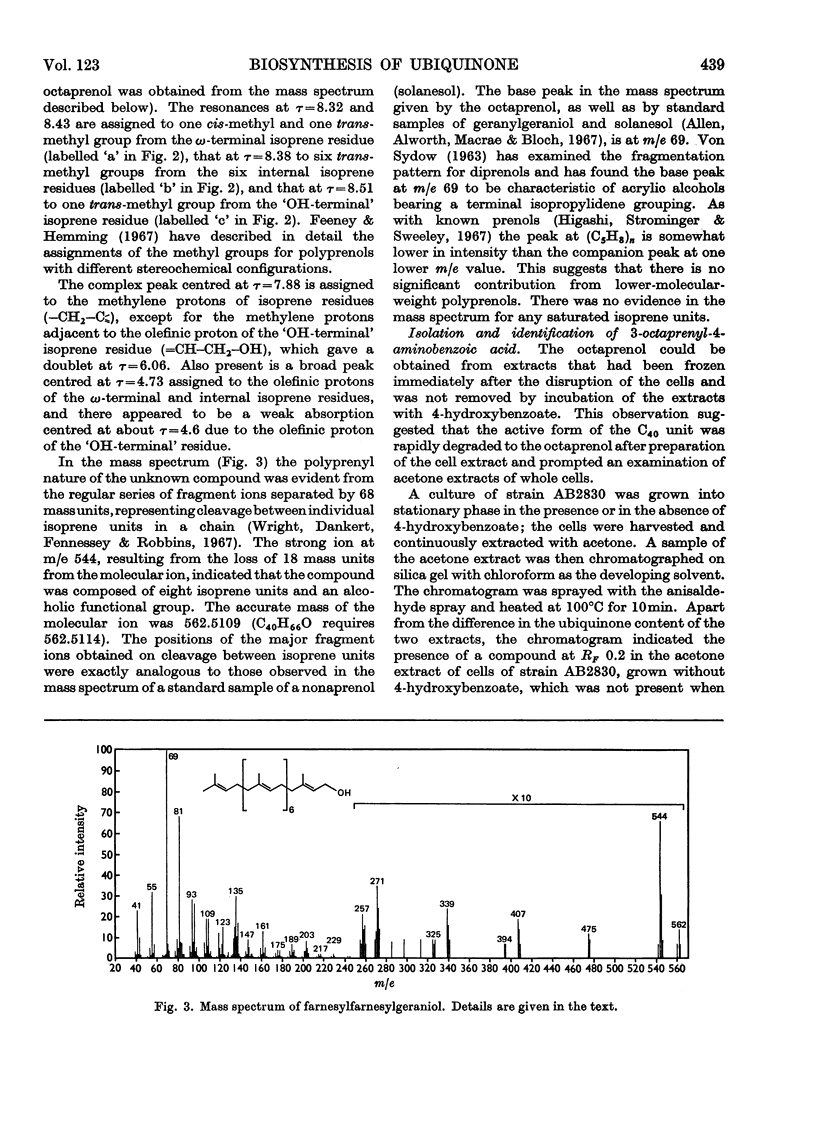
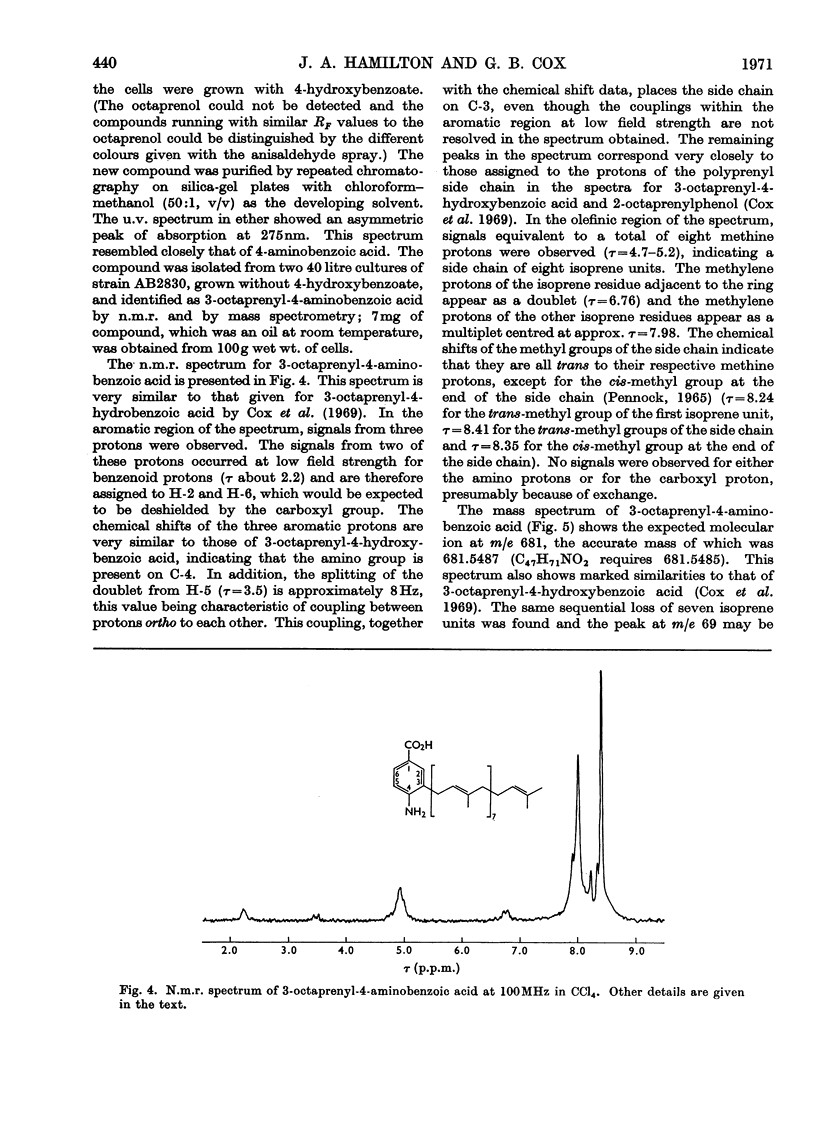
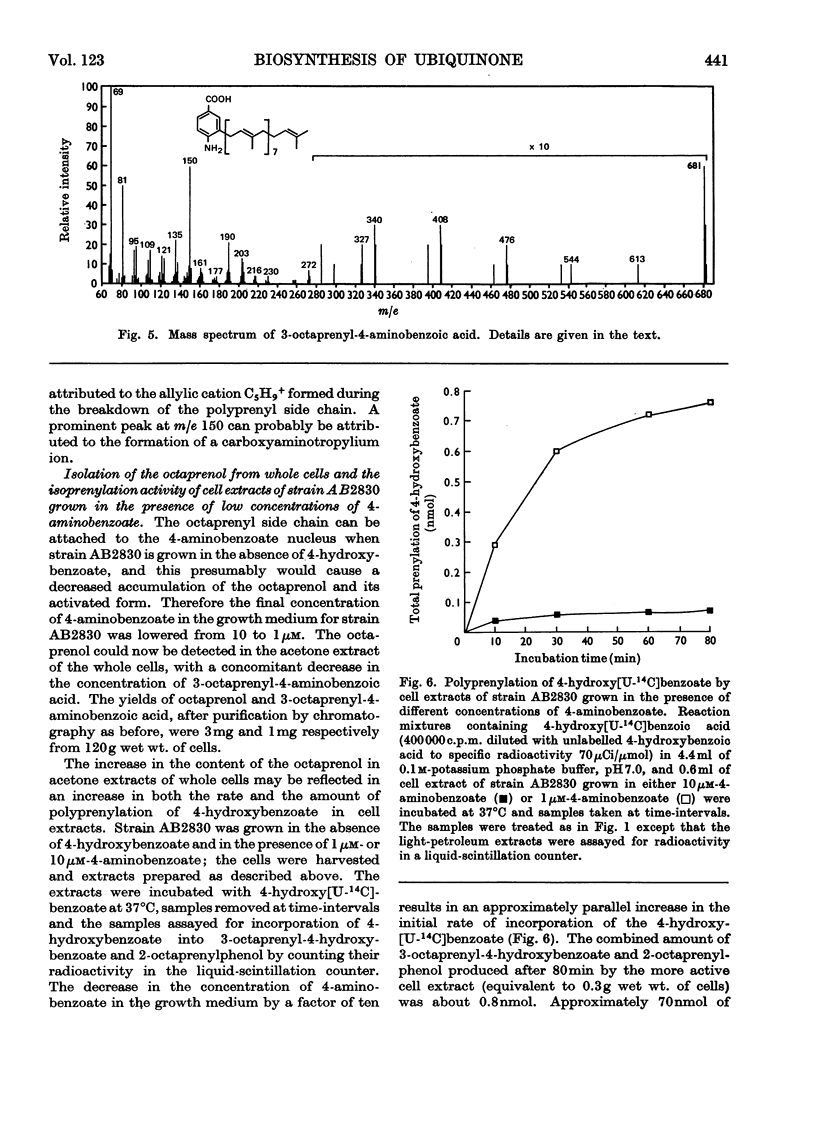
Cell extracts of a multiple aromatic auxotroph of Escherichia coli K-12, strain AB2830, grown in the absence of precursors of the quinone rings of the ubiquinone and menaquinone molecules, converted 4-hydroxy[U-14C]benzoate into a mixture of 3-octaprenyl-4-hydroxybenzoate and 2-octaprenylphenol. An octaprenol, farnesylfarnesylgeraniol, was isolated from such cell extracts and characterized by n.m.r. and mass spectroscopy. Neither the octaprenol, nor polyprenylation of 4-hydroxy[U-14C]benzoate, could be detected in cell extracts of strain AB2830 grown in the presence of 0.1mm-4-hydroxybenzoate. It was concluded that, in the biosynthesis of ubiquinone, the polyprenyl side chain is added to 4-hydroxybenzoate as a C40 unit, the active form of which is converted by cell extracts into farnesylfarnesylgeraniol. The multiple aromatic auxotroph, when grown in the absence of 4-hydroxybenzoate but in the presence of 4-aminobenzoate, converted the latter compound into 3-octaprenyl-4-aminobenzoate. This compound was isolated from whole cells and characterized by n.m.r. and mass spectroscopy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen C. M., Alworth W., Macrae A., Bloch K. A long chain terpenyl pyrophosphate synthetase from Micrococcus lysodeikticus. J Biol Chem. 1967 Apr 25;242(8):1895–1902. [PubMed] [Google Scholar]
- Cox G. B., Gibson F. The role of shikimic acid in the biosynthesis of vitamin K2. Biochem J. 1966 Jul;100(1):1–6. doi: 10.1042/bj1000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Young I. G., McCann L. M., Gibson F. Biosynthesis of ubiquinone in Escherichia coli K-12: location of genes affecting the metabolism of 3-octaprenyl-4-hydroxybenzoic acid and 2-octaprenylphenol. J Bacteriol. 1969 Aug;99(2):450–458. doi: 10.1128/jb.99.2.450-458.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D. Inhibition of Escherichia coli by p-aminobenzoic acid and its reversal by p-hydroxybenzoic acid. J Exp Med. 1951 Sep;94(3):243–254. doi: 10.1084/jem.94.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney J., Hemming F. W. Nuclear magnetic resonance spectrometry of naturally occurring polyprenols. Anal Biochem. 1967 Jul;20(1):1–15. doi: 10.1016/0003-2697(67)90258-8. [DOI] [PubMed] [Google Scholar]
- Friis P., Nilsson J. L., Daves G. D., Jr, Folkers K. New multiprenylquinones in the biosynthesis of ubiquinone. Biochem Biophys Res Commun. 1967 Aug 7;28(3):324–327. doi: 10.1016/0006-291x(67)90312-9. [DOI] [PubMed] [Google Scholar]
- Gibson M. I., Gibson F. Preliminary studies on the isolation and metabolism of an intermediate in aromatic biosynthesis: chorismic acid. Biochem J. 1964 Feb;90(2):248–256. doi: 10.1042/bj0900248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R. K., White D. C. Formation of vitamin K2 isoprenologues by Staphylococcus aureus. J Bacteriol. 1969 Nov;100(2):573–578. doi: 10.1128/jb.100.2.573-578.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y., Strominger J. L., Sweeley C. C. Structure of a lipid intermediate in cell wall peptidoglycan synthesis: a derivative of a C55 isoprenoid alcohol. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1878–1884. doi: 10.1073/pnas.57.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- OLSEN R. K., SMITH J. L., DAVES G. D., MOORE H. W., FOLKERS K., PARSON W. W., RUDNEY H. 2-DECAPRENYLPHENOL, BIOSYNTHETIC PRECURSOR OF UBIQUINONE-10. J Am Chem Soc. 1965 May 20;87:2298–2300. doi: 10.1021/ja01088a045. [DOI] [PubMed] [Google Scholar]
- Olsen R. K., Daves G. D., Jr, Moore H. W., Folkers K., Parson W. W., Rudney H. 2-multiprenylphenols and 2-decaprenyl-6-methoxyphenol, biosynthetic precursors of ubiquinones. J Am Chem Soc. 1966 Dec 20;88(24):5919–5923. doi: 10.1021/ja00976a036. [DOI] [PubMed] [Google Scholar]
- PARSON W. W., RUDNEY H. THE BIOSYNTHESIS OF THE BENZOQUINONE RING OF UBIQUINONE FROM P-HYDROXYBENZALDEHYDE AND P-HYDROXYBENZOIC ACID IN RAT KIDNEY, AZOTOBACTER VINELANDII, AND BAKER'S YEAST. Proc Natl Acad Sci U S A. 1964 Mar;51:444–450. doi: 10.1073/pnas.51.3.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher M., Lennarz W. J., Sweeley C. C. The biosynthesis of mannosyl-1-phosphoryl-polyisoprenol in Micrococcus lysodeikticus and its role in mannan synthesis. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1313–1320. doi: 10.1073/pnas.59.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A., Dankert M., Fennessey P., Robbins P. W. Characterization of a polyisoprenoid compound functional in O-antigen biosynthesis. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1798–1803. doi: 10.1073/pnas.57.6.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]


