Abstract
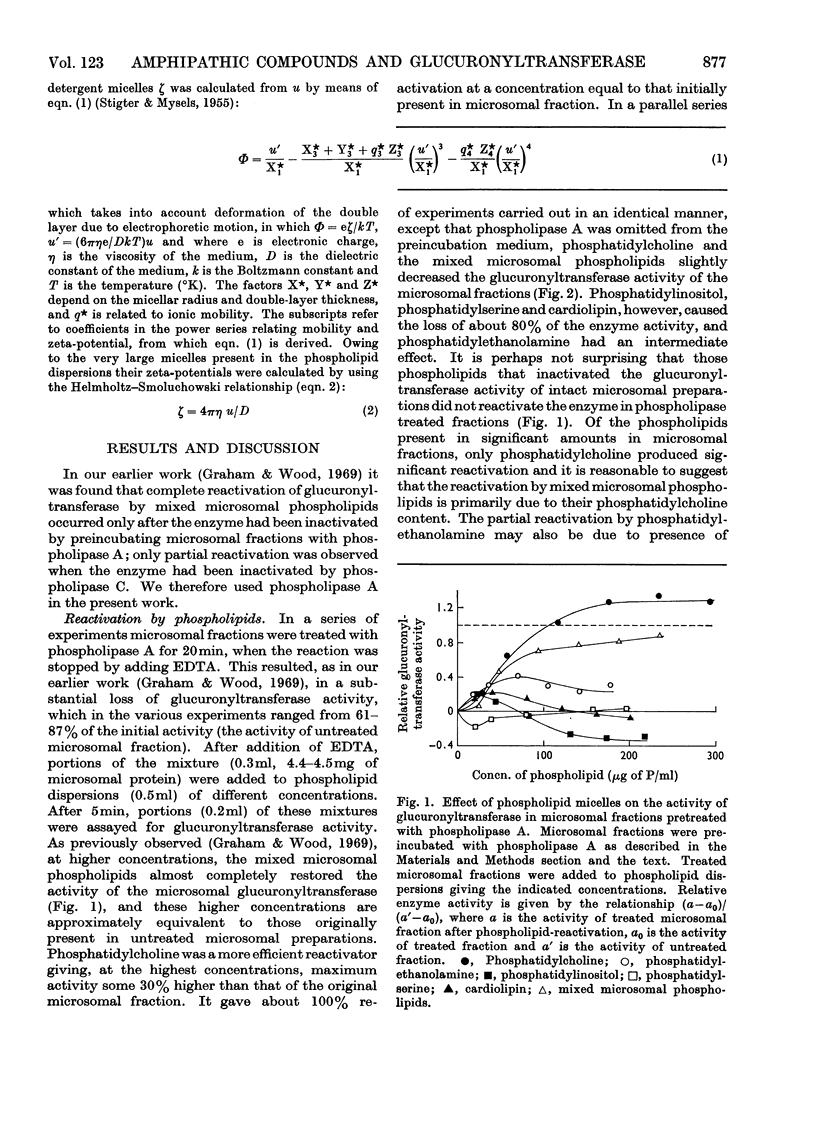
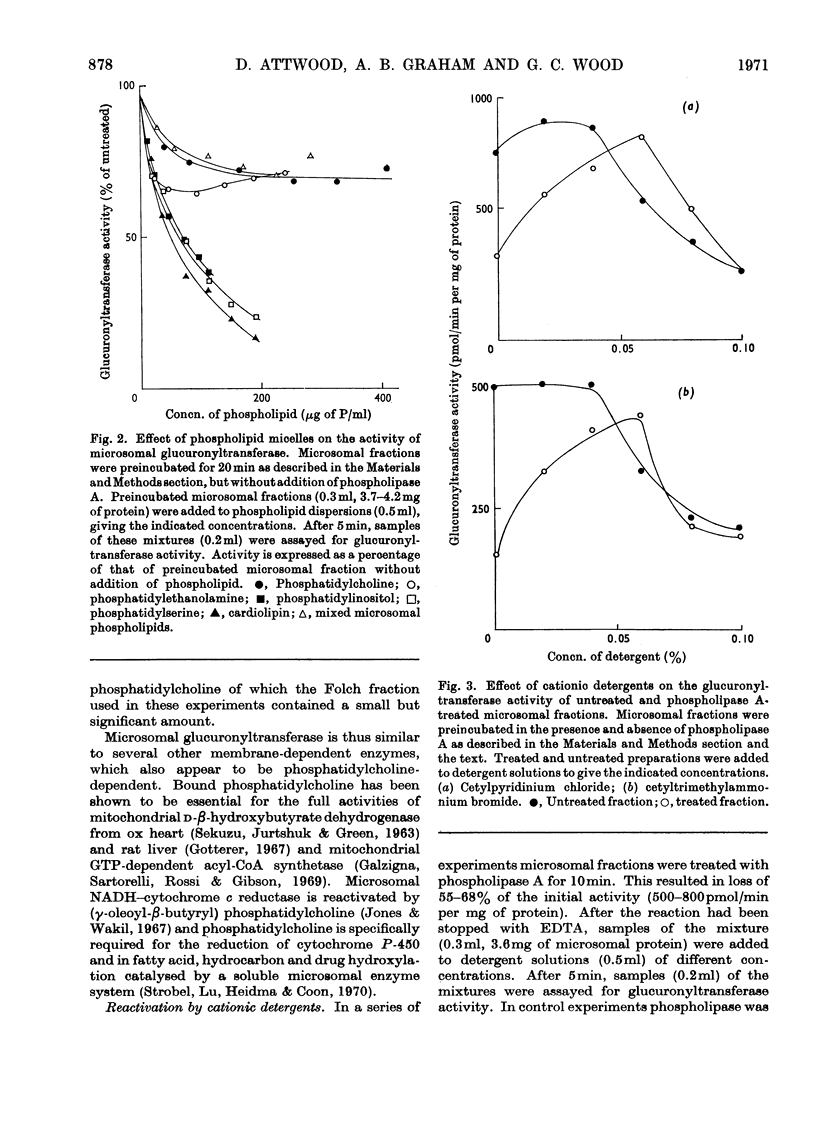
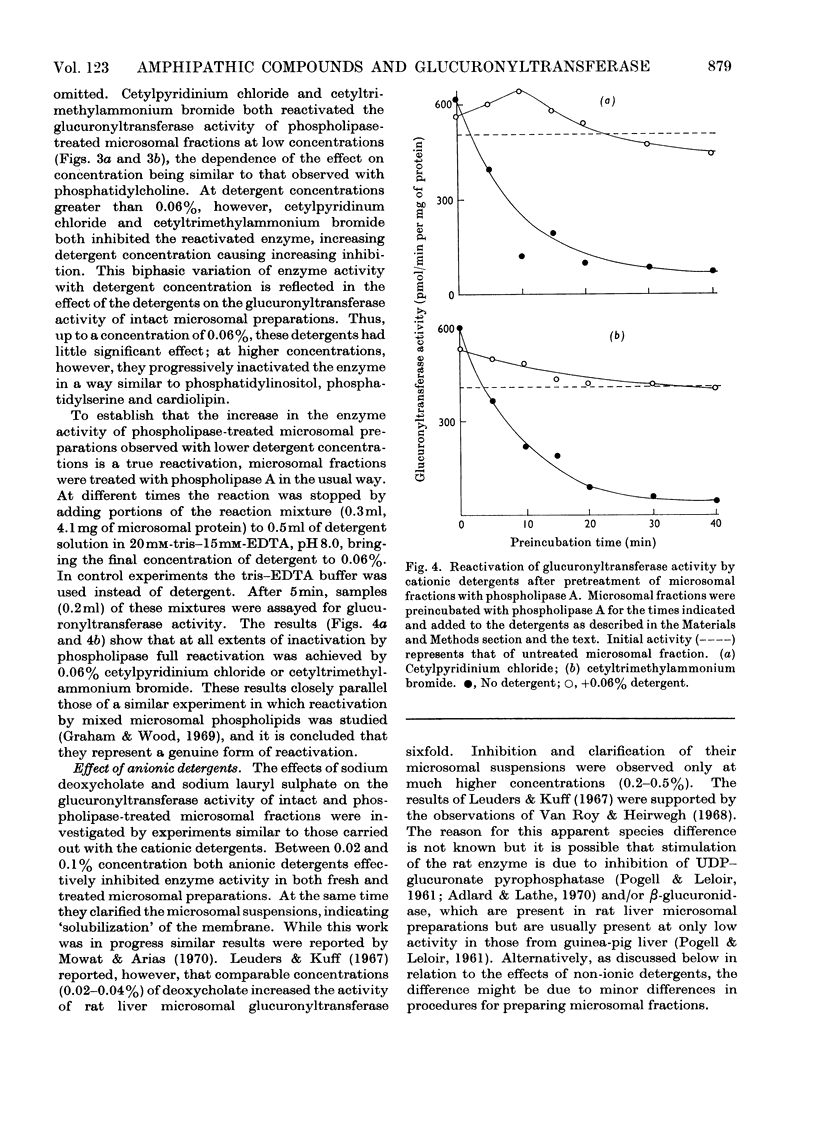
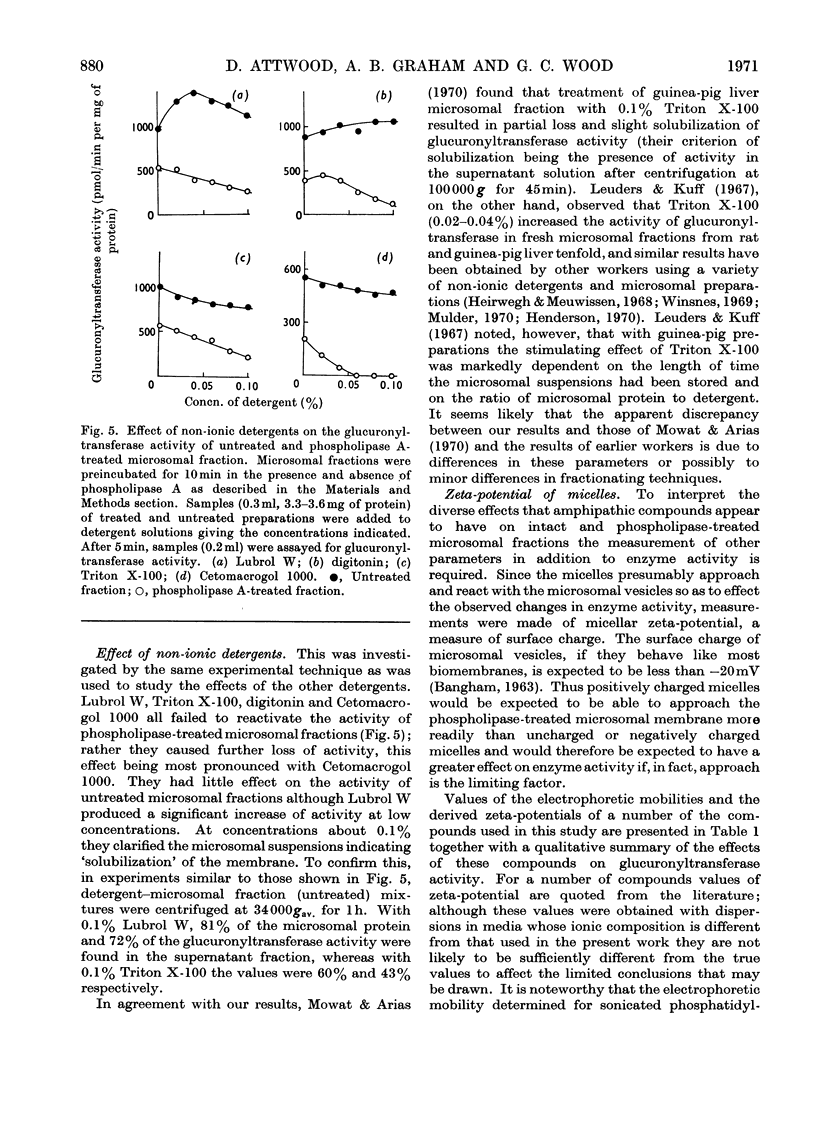
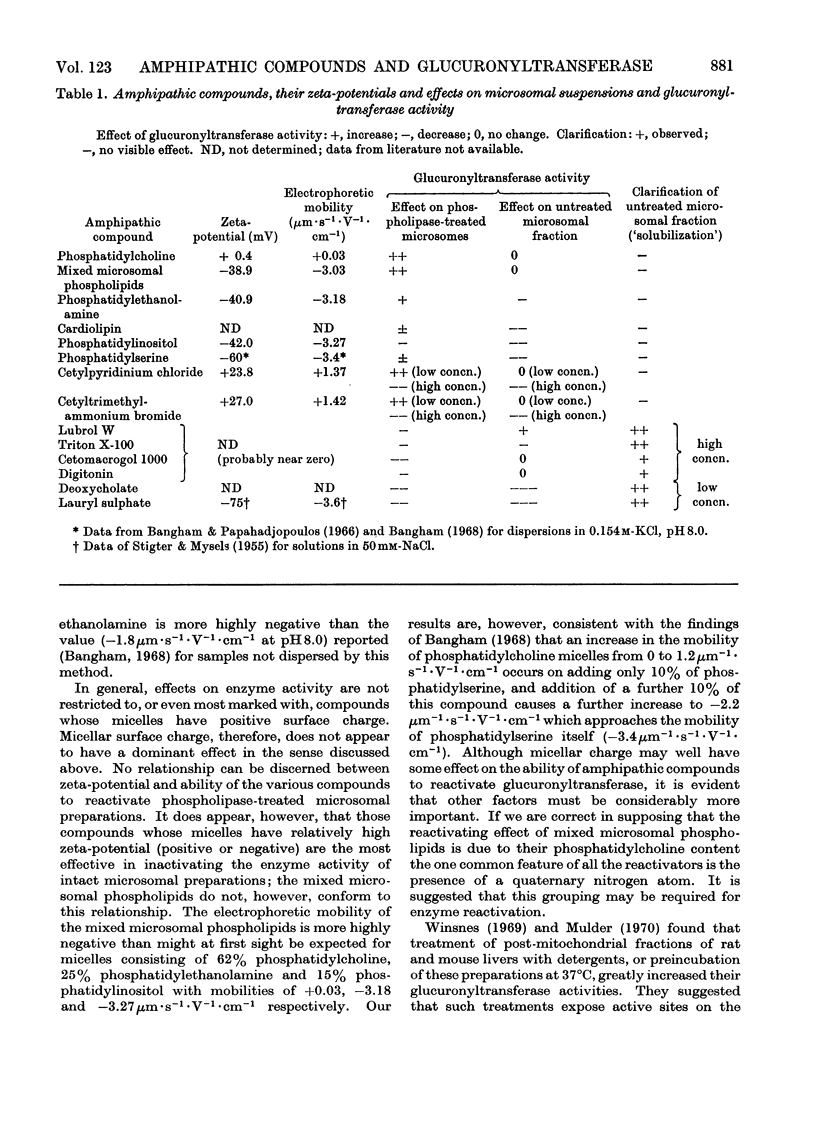
Specific degradation of the phospholipid membrane of guinea-pig liver microsomal fraction with phospholipase A inactivated glucuronyltransferase. The inactivation was reversed by phosphatidylcholine and mixed microsomal phospholipid micelles at concentrations similar to those present in intact microsomal preparations. The other commonly occurring phospholipids did not reactivate phospholipase A-treated enzyme. Since the mixed microsomal phospholipids consisted mainly of phosphatidylcholine, it is concluded that the reactivation by phospholipids is phosphatidylcholine-specific. Reactivation was also achieved by low concentrations of the cationic detergents cetylpyridinium chloride and cetyltrimethylammonium bromide. Higher concentrations of these detergents inactivated the glucuronyltransferase activity of intact and phospholipase A-treated microsomal fractions. Anionic detergents were potent inactivators of the glucuronyltransferase activity of untreated and phospholipase A-treated microsomal fractions, whereas non-ionic detergents had little effect on the activity of either preparation. Measurements of the zeta-potentials of the micellar species used in this study showed that no obvious relationship existed between the zeta-potentials and the ability to reactivate glucuronyltransferase. However, high positive or negative zeta-potentials were correlated with the ability of the amphipathic compound to inactivate glucuronyltransferase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlard B. P., Lathe G. H. The effect of steroids and nucleotidesoon solubilized bilirubin uridine diphosphate-glucuronyltransferase. Biochem J. 1970 Sep;119(3):437–445. doi: 10.1042/bj1190437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANGHAM A. D. PHYSICAL STRUCTURE AND BEHAVIOR OF LIPIDS AND LIPID ENZYMES. Adv Lipid Res. 1963;1:65–104. doi: 10.1016/b978-1-4831-9937-5.50008-9. [DOI] [PubMed] [Google Scholar]
- Bangham A. D. Membrane models with phospholipids. Prog Biophys Mol Biol. 1968;18:29–95. doi: 10.1016/0079-6107(68)90019-9. [DOI] [PubMed] [Google Scholar]
- Bangham A. D., Papahadjopoulos D. Biophysical properties of phospholipids. I. Interaction of phosphatidylserine monolayers with metal ions. Biochim Biophys Acta. 1966 Sep 5;126(1):181–184. doi: 10.1016/0926-6585(66)90052-5. [DOI] [PubMed] [Google Scholar]
- Galzigna L., Sartorelli L., Rossi C. R., Gibson D. M. Lecithin-protein interaction in the GTP-dependent acyl-CoA synthase. Lipids. 1969 Nov;4(6):459–461. doi: 10.1007/BF02531024. [DOI] [PubMed] [Google Scholar]
- Gotterer G. S. Rat liver D-beta-hydroxybutyrate dehydrogenase. II. Lipid requirement. Biochemistry. 1967 Jul;6(7):2147–2152. doi: 10.1021/bi00859a036. [DOI] [PubMed] [Google Scholar]
- Graham A. B., Wood G. C. The phospholipid-dependence of UDP-glucuronyltransferase. Biochem Biophys Res Commun. 1969 Nov 6;37(4):567–575. doi: 10.1016/0006-291x(69)90846-8. [DOI] [PubMed] [Google Scholar]
- Green D. E., Tzagoloff A. Role of lipids in the structure and function of biological membranes. J Lipid Res. 1966 Sep;7(5):587–602. [PubMed] [Google Scholar]
- HOLLMANN S., TOUSTER O. Alterations in tissue levels of uridine diphosphate glucose dehydrogenase, uridine diphosphate glucuronic acid pyrophosphatase and glucuronyl transferase induced by substances influencing the production of ascorbic acid. Biochim Biophys Acta. 1962 Aug 13;62:338–352. doi: 10.1016/0006-3002(62)90048-3. [DOI] [PubMed] [Google Scholar]
- Heirwegh K. P., Meuwissen J. A. Activation in vitro and solubilization of glucuronyltransferase (assayed with bilirubin as acceptor) with digitonin. Biochem J. 1968 Dec;110(3):31P–32P. doi: 10.1042/bj1100031pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. T. Activation in vitro of rat hepatic UDP-glucuronyltransferase by ultrasound. Life Sci II. 1970 May 8;9(9):511–518. doi: 10.1016/0024-3205(70)90351-6. [DOI] [PubMed] [Google Scholar]
- ISSELBACHER K. J., CHRABAS M. F., QUINN R. C. The solubilization and partial purification of a glucuronyl transferase from rabbit liver microsomes. J Biol Chem. 1962 Oct;237:3033–3036. [PubMed] [Google Scholar]
- Jones P. D., Wakil S. J. A requirement for phospholipids by the microsomal reduced diphosphopyridine nucleotide-cytochrome c reductase. J Biol Chem. 1967 Nov 25;242(22):5267–5273. [PubMed] [Google Scholar]
- Leventer L. L., Buchanan J. L., Ross J. E., Tapley D. F. Solubilization of N-glucuronyl transferase. Biochim Biophys Acta. 1965 Nov 22;110(2):428–430. doi: 10.1016/s0926-6593(65)80052-2. [DOI] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Spontaneous and detergent activation of a glucuronyltransferase in vitro. Arch Biochem Biophys. 1967 Apr;120(1):198–203. doi: 10.1016/0003-9861(67)90614-5. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R. A FAST, SIMPLE AND RELIABLE METHOD FOR THE MICRODETERMINATION OF PHOSPHORUS IN BIOLOGICAL MATERIALS. Anal Biochem. 1964 Feb;7:218–224. doi: 10.1016/0003-2697(64)90231-3. [DOI] [PubMed] [Google Scholar]
- Martonosi A., Donley J., Halpin R. A. Sarcoplasmic reticulum. 3. The role of phospholipids in the adenosine triphosphatase activity and Ca++ transport. J Biol Chem. 1968 Jan 10;243(1):61–70. [PubMed] [Google Scholar]
- Morrison W. R. The distribution of phospholipids in some mammalian milks. Lipids. 1968 Jan;3(1):101–103. doi: 10.1007/BF02530978. [DOI] [PubMed] [Google Scholar]
- Mowat A. P., Arias I. M. Partial purification of hepatic UDP-glucuronyltransferase. Studies of some of its properties. Biochim Biophys Acta. 1970 Jul 15;212(1):65–78. doi: 10.1016/0005-2744(70)90179-8. [DOI] [PubMed] [Google Scholar]
- Mulder G. J. The effect of phenobarbital on the submicrosomal distribution of uridine diphosphate glucuronyltransferase from rat liver. Biochem J. 1970 Apr;117(2):319–324. doi: 10.1042/bj1170319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POGELL B. M., LELOIR L. F. Nucleotide activation of liver microsomal glucuronidation. J Biol Chem. 1961 Feb;236:293–298. [PubMed] [Google Scholar]
- Palatini P., Bruni A. Reversal by phospholipids of the oligomycin induced inhibition of membrane associated adenosintriphosphatases. Biochem Biophys Res Commun. 1970 Jul 13;40(1):186–191. doi: 10.1016/0006-291x(70)91064-8. [DOI] [PubMed] [Google Scholar]
- Rao G. S., Rao M. L., Breuer H. Partial purification and kinetics of oestriol 16 alpha-glucuronyltransferase from the cytosol fraction of human liver. Biochem J. 1970 Jul;118(4):625–634. doi: 10.1042/bj1180625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEKUZU I., JURTSHUK P., Jr, GREEN D. E. Studies on the electron transfer system. LI. Isolation and characterization of the D-(--)-beta-hydroxybutyric apodehydrogenase from beef heart mitochondria. J Biol Chem. 1963 Mar;238:975–982. [PubMed] [Google Scholar]
- Strobel H. W., Lu A. Y., Heidema J., Coon M. J. Phosphatidylcholine requirement in the enzymatic reduction of hemoprotein P-450 and in fatty acid, hydrocarbon, and drug hydroxylation. J Biol Chem. 1970 Sep 25;245(18):4851–4854. [PubMed] [Google Scholar]
- Tobari J. Requirement of flavin adenine dinucleotide and phospholipid for the activity of malate dehydrogenase from Mycobacterium avium. Biochem Biophys Res Commun. 1964 Feb 18;15(1):50–54. doi: 10.1016/0006-291x(64)90101-9. [DOI] [PubMed] [Google Scholar]
- Van Roy F. P., Heirwegh K. P. Determination of bilirubin glucuronide and assay of glucuronyltransferase with bilirubin as acceptor. Biochem J. 1968 Apr;107(4):507–518. doi: 10.1042/bj1070507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsnes A. Studies on the activation in vitro of glucuronyltransferase. Biochim Biophys Acta. 1969 Nov 4;191(2):279–291. doi: 10.1016/0005-2744(69)90247-2. [DOI] [PubMed] [Google Scholar]


