Abstract
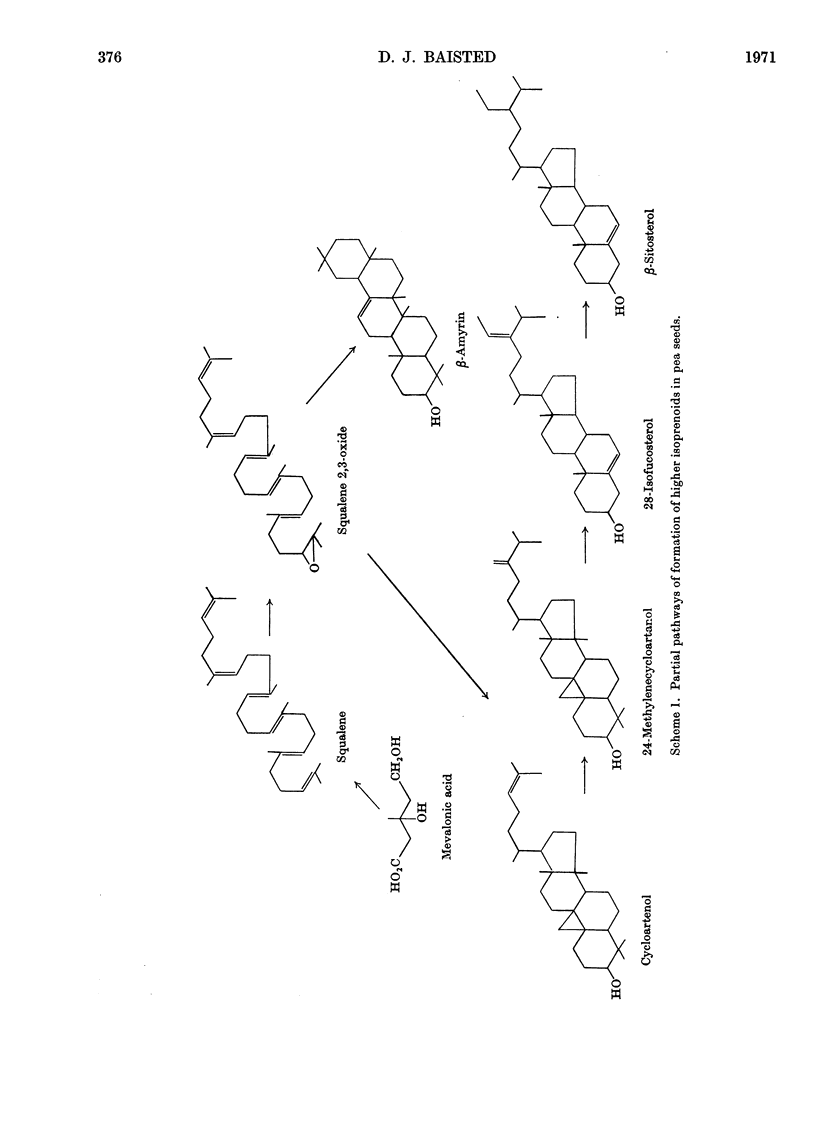
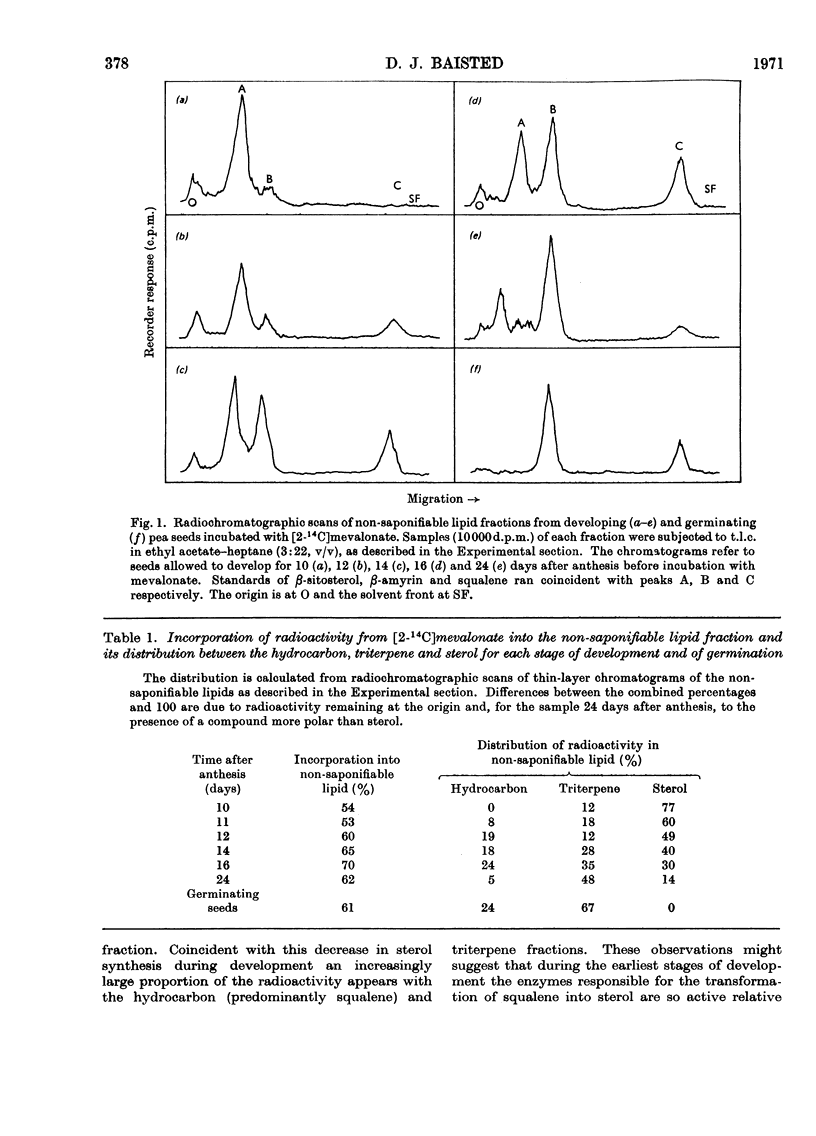
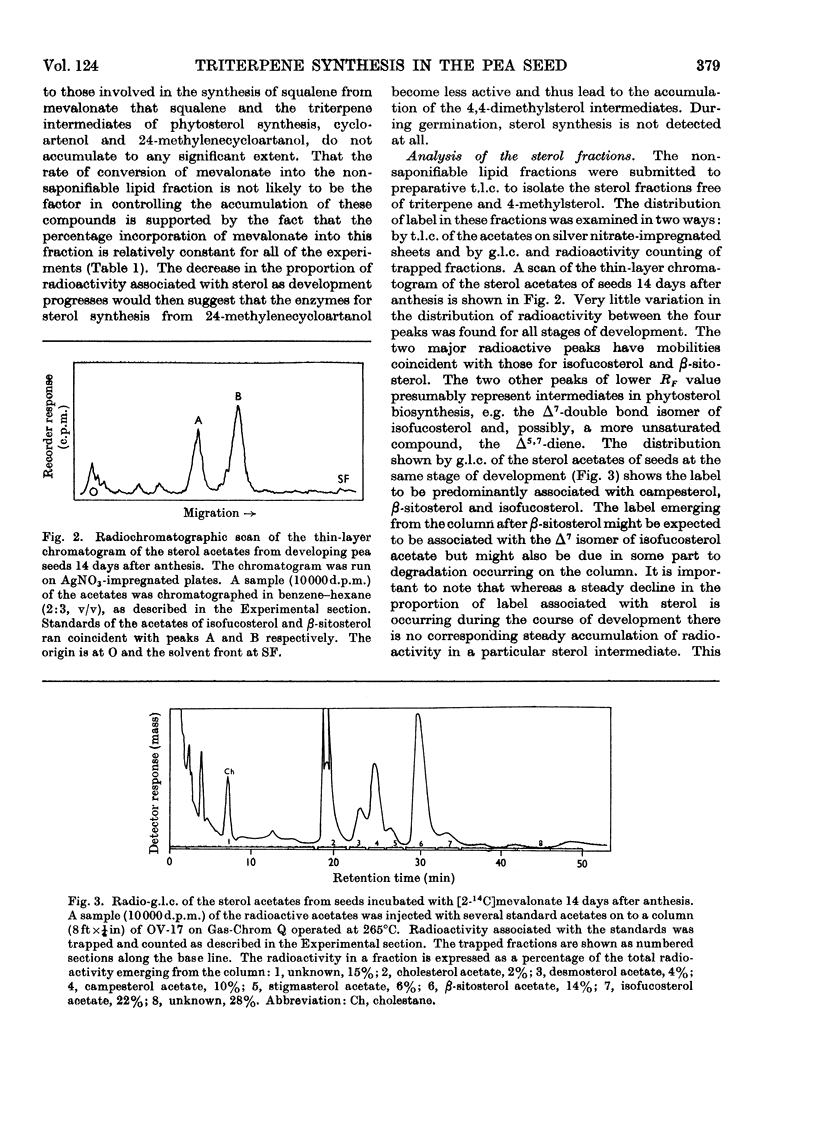
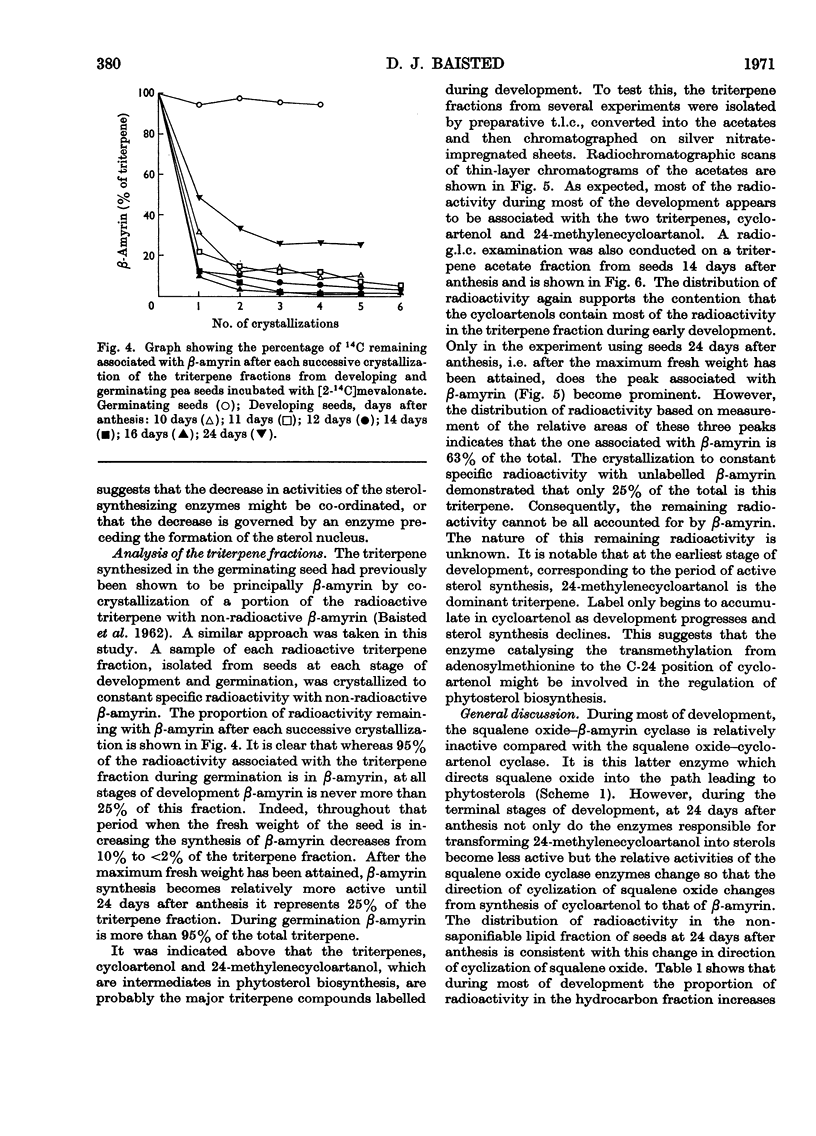
Developing and germinating pea seeds were compared with respect to their capacity to incorporate mevalonate into sterols and triterpenes. The capacity for sterol synthesis is greatest in the least mature fruits and decreases during their development. Label is shown, by gas–liquid chromatography and counting the radioactivity of trapped fractions, to be associated with campesterol, β-sitosterol and isofucosterol. During early stages of germination sterol synthesis is insignificant. The triterpene fraction becomes heavily labelled during both development and germination. The label is associated almost exclusively with β-amyrin during germination but with cycloartenol and 24-methylenecycloartanol during development. It is only in the terminal stages of maturation that β-amyrin becomes significantly labelled. At the same time an unidentified radioactive polar compound appears. The possible significance of the appearance of this polar compound and the regulation of the synthesis of these higher terpenoids is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAISTED D. J., CAPSTACK E., Jr, NES W. R. The biosynthesis of beta-amyrin and beta-sitosterol in germinating seeds of Pisum sativum. Biochemistry. 1962 May 25;1:537–541. doi: 10.1021/bi00909a027. [DOI] [PubMed] [Google Scholar]
- BANGHAM A. D., HORNE R. W., GLAUERT A. M., DINGLE J. T., LUCY J. A. Action of saponin on biological cell membranes. Nature. 1962 Dec 8;196:952–955. doi: 10.1038/196952a0. [DOI] [PubMed] [Google Scholar]
- Bennett R. D., Lieber E. R., Heftmann E. Time Course of Steroid Biosynthesis and Metabolism in Haplopappus heterophyllus. Plant Physiol. 1967 Jul;42(7):973–976. doi: 10.1104/pp.42.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAPSTACK E., Jr, BAISTED D. J., NEWSCHWANDER W. W., BLONDIN G., ROSIN N. L., NES W. R. The biosynthesis of squalene in germinating seeds of Pisum sativum. Biochemistry. 1962 Nov;1:1178–1183. doi: 10.1021/bi00912a033. [DOI] [PubMed] [Google Scholar]
- Coolbaugh R. C., Moore T. C. Apparent changes in rate of kaurene biosynthesis during the development of pea seeds. Plant Physiol. 1969 Sep;44(9):1364–1367. doi: 10.1104/pp.44.9.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner P., Varner J. E., Wray J. L. Environmental or developmental changes cause many enzyme activities of higher plants to rise or fall. Science. 1969 Jul 25;165(3891):358–367. doi: 10.1126/science.165.3891.358. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Finkelstein A. Membrane biochemistry. Annu Rev Biochem. 1968;37:463–496. doi: 10.1146/annurev.bi.37.070168.002335. [DOI] [PubMed] [Google Scholar]
- Shechter I., Sweat F. W., Bloch K. Comparative properties of 2,3-oxidosqualene-lanosterol cyclase from yeast and liver. Biochim Biophys Acta. 1970 Dec 16;220(3):463–468. doi: 10.1016/0005-2744(70)90277-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Lin K., Bloch K. Some properties of the microsomal 2,3-oxidosqualene sterol cyclase. Proc Natl Acad Sci U S A. 1969 May;63(1):110–117. doi: 10.1073/pnas.63.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]


