Abstract
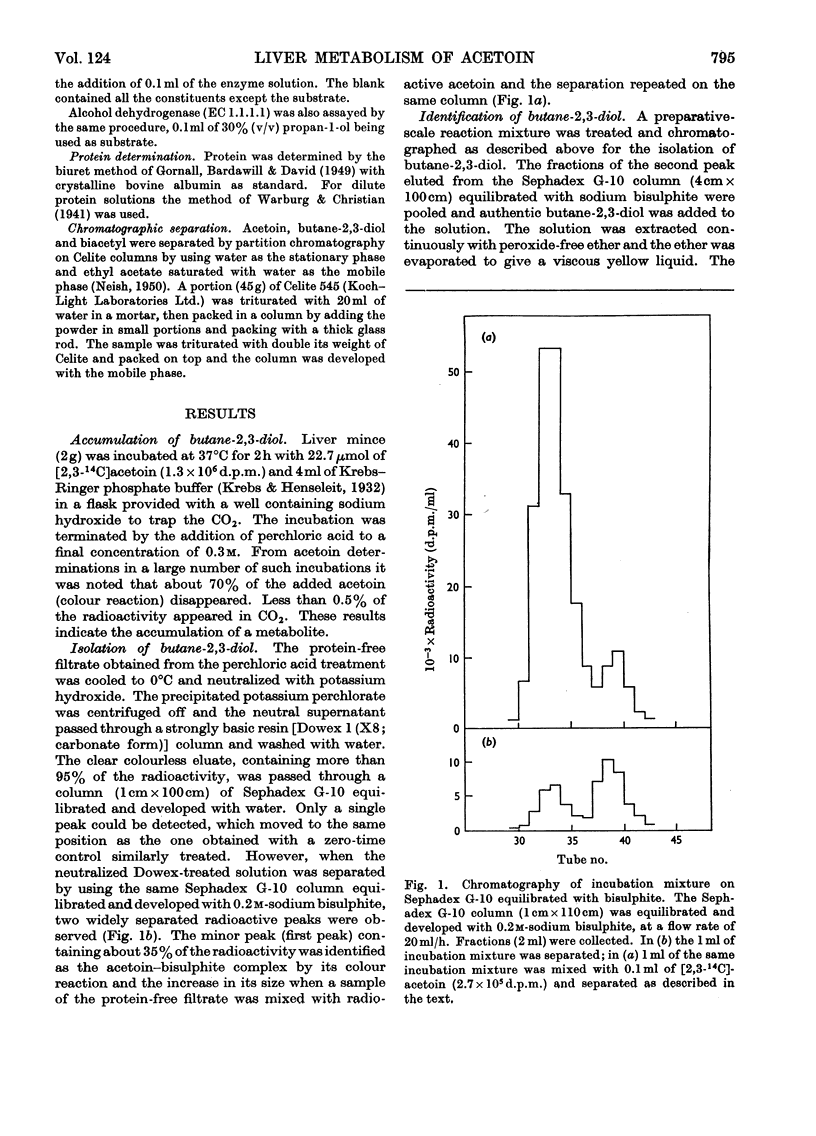
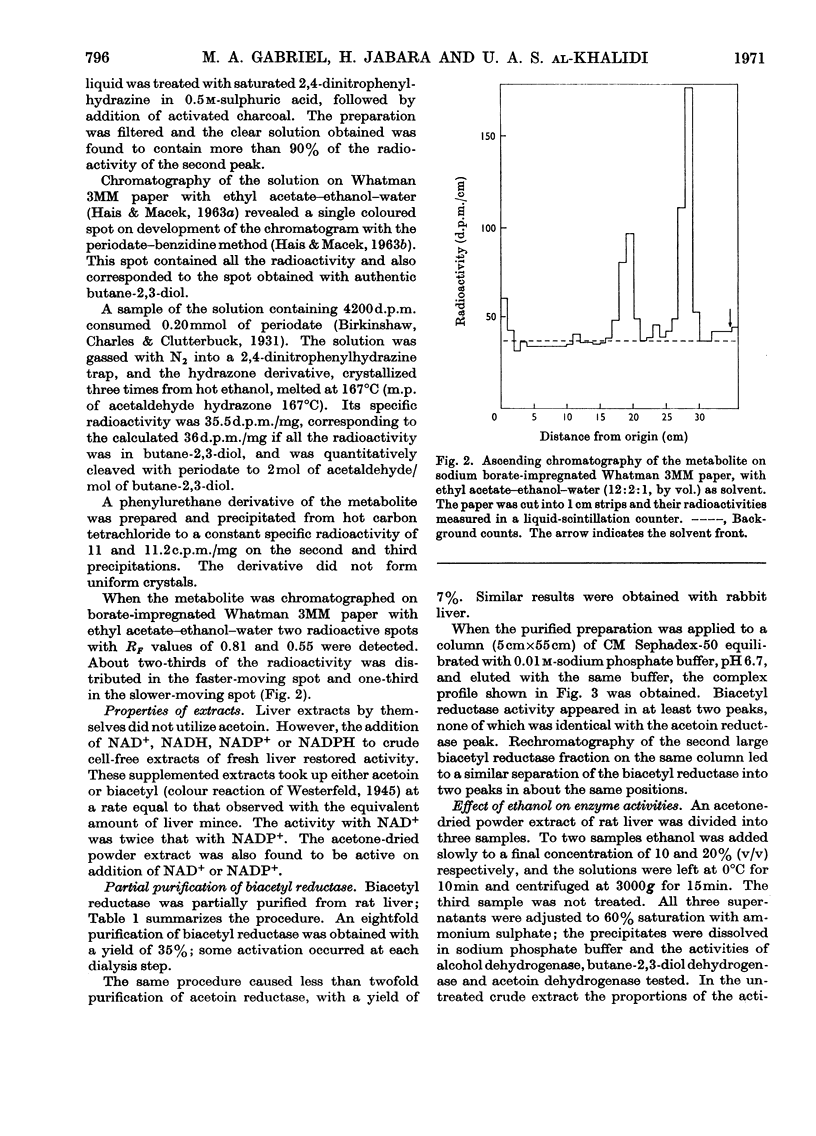
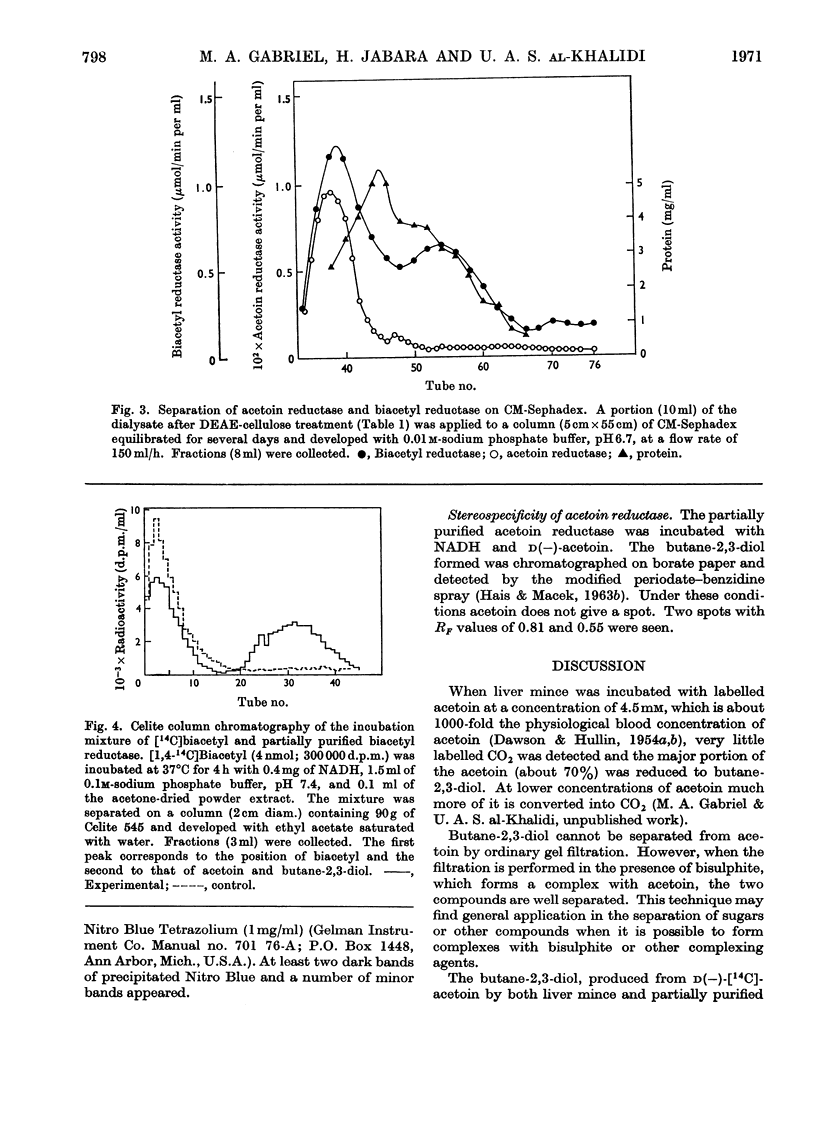
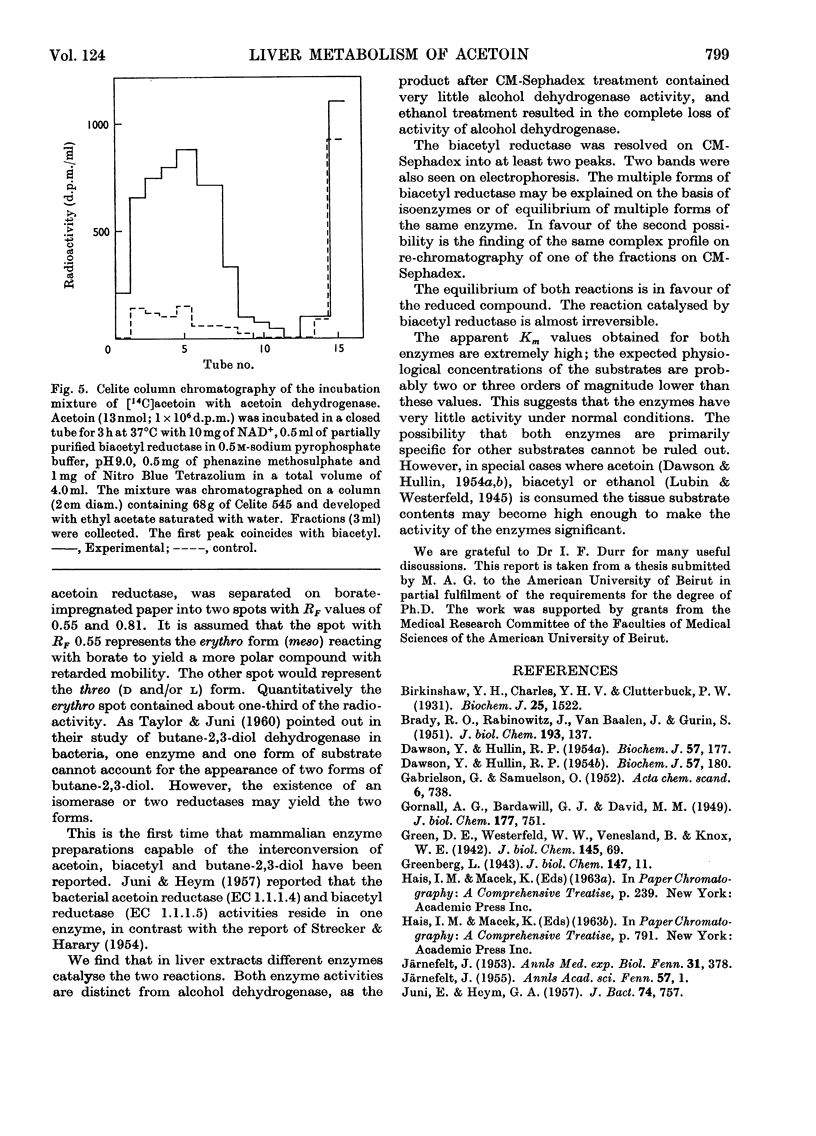
1. [14C]Acetoin was enzymically synthesized from [14C]pyruvate with a pyruvate decarboxylase preparation. Its optical activity was [α]20d−78°. 2. Large amounts (1000-fold higher than physiological concentrations) of acetoin were incubated with rat liver mince. Acetoin disappeared but very little 14CO2 was evolved. A compound accumulated, which was purified and identified as butane-2,3-diol. Chromatography on borate-impregnated paper indicated the presence of both the erythro and threo forms. 3. Liver extracts capable of interconverting biacetyl, acetoin and butane-2,3-diol were obtained. These interconversions were catalysed by two different enzymes: acetoin dehydrogenase (EC 1.1.1.5) and butane-2,3-diol dehydrogenase (EC 1.1.1.4), previously identified in bacteria. Both required NAD+ or NADP+ as cofactors and were different from alcohol dehydrogenase. The equilibrium in both cases favoured the more reduced compound. 4. The activity of butane-2,3-diol dehydrogenase was decreased by dialysis against EDTA: the addition of Co2+, Cu2+, Zn2+ and other bivalent metal ions restored activity. 5. Biacetyl reductase was resolved into multiple forms by CM-Sephadex chromatography and electrophoresis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADY R. O., RABINOWITZ J., VAN BAALEN J., GURIN S. The synthesis of radioactive cholesterol and fatty acids in vitro. II. A further study of precursors. J Biol Chem. 1951 Nov;193(1):137–143. [PubMed] [Google Scholar]
- Birkinshaw J. H., Charles J. H., Clutterbuck P. W. Studies in the biochemistry of micro-organisms: Quantitative examination by the carbon balance sheet method of the types of products formed from glucose by species of bacteria. Biochem J. 1931;25(5):1522–1539. doi: 10.1042/bj0251522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON J., HULLIN R. P. Metabolism of acetoin. I. The formation and utilization of acetoin and butane-2:3-diol in the decerebrated cat. Biochem J. 1954 Jun;57(2):177–180. doi: 10.1042/bj0570177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON J., HULLIN R. P. Metabolism of acetoin. II. Metabolic conversions of acetoin, pyruvate and acetate by rabbit-kidney tissue dispersions. Biochem J. 1954 Jun;57(2):180–185. doi: 10.1042/bj0570180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARNEFELT J. Metabolism of acetoin and diacetyl in liver tissue; some observations. Ann Med Exp Biol Fenn. 1953;31(4):378–384. [PubMed] [Google Scholar]
- JUNI E., HEYM G. A. Cyclic pathway for the bacterial dissimilation of 2,3-butanediol, acetylmethylcarbinol, and diacetyl. III. A comparative study of 2,3-butanediol dehydrogenases from various microorganisms. J Bacteriol. 1957 Dec;74(6):757–767. doi: 10.1128/jb.74.6.757-767.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRECKER H. J., HARARY I. Bacterial butylene glycol dehydrogenase and diacetyl reductase. J Biol Chem. 1954 Nov;211(1):263–270. [PubMed] [Google Scholar]
- TAYLOR M. B., JUNI E. Stereoisomeric specificities of 2,3-butanediol dehydrogenases. Biochim Biophys Acta. 1960 Apr 22;39:448–457. doi: 10.1016/0006-3002(60)90197-9. [DOI] [PubMed] [Google Scholar]


