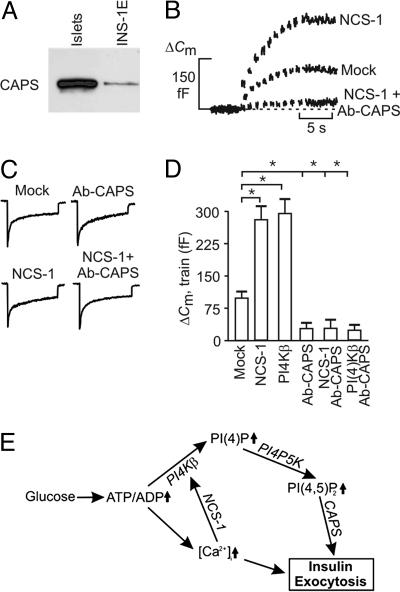
Fig. 5.
CAPS is involved in NCS-1- and PI4Kβ-dependent exocytosis. (A) Western blot showing expression of CAPS in NMRI mouse islets and INS-1E cells. (B) Capacitance increases elicited by trains of 10 500-ms depolarizations in single mouse β cells transfected with EGFP (mock) or NCS-1. The experiments commenced 2 min after establishment of the standard whole-cell configuration. Increases in cell capacitance also were recorded in cells expressing NCS-1 after inclusion of an antibody against CAPS (anti-CAPS, 1.5 mg/ml) in the pipette-filling solution (NCS-1 plus Ab-CAPS). All experiments were conducted in the presence of 20 mM glucose. (C) Whole-cell Ca2+ currents evoked by the first pulse in a train of membrane depolarizations in mock- and NCS-1-transfected cells in the absence and presence of antibody against CAPS (Ab-CAPS). (D) Histogram summarizing exocytosis elicited by trains of depolarizations applied 2 min after establishment of the whole-cell configuration in cells transfected with EGFP (mock), NCS-1, or PI4Kβ in the absence and presence of an antibody against CAPS (Ab-CAPS, 1.5 mg/ml). Data are mean values ± SEM of five to seven experiments. *, P < 0.01. (E) Model for regulation of exocytosis by NCS-1 and PI4Kβ after glucose stimulation in pancreatic β cells. High glucose raises the ATP/ADP ratio. Increased ATP/ADP leads to membrane depolarization and, ultimately, to an increase in cytoplasmic Ca2+ concentration, [Ca2+]i. Both elevated ATP/ADP (directly) and [Ca2+]i (through NCS-1) stimulate PI4Kβ activity. PI(4)P and PI(4,5)P2, formed upon the sequential stimulation of PI4Kβ and PI4P 5-kinase, enhance Ca2+-induced insulin exocytosis.