Abstract
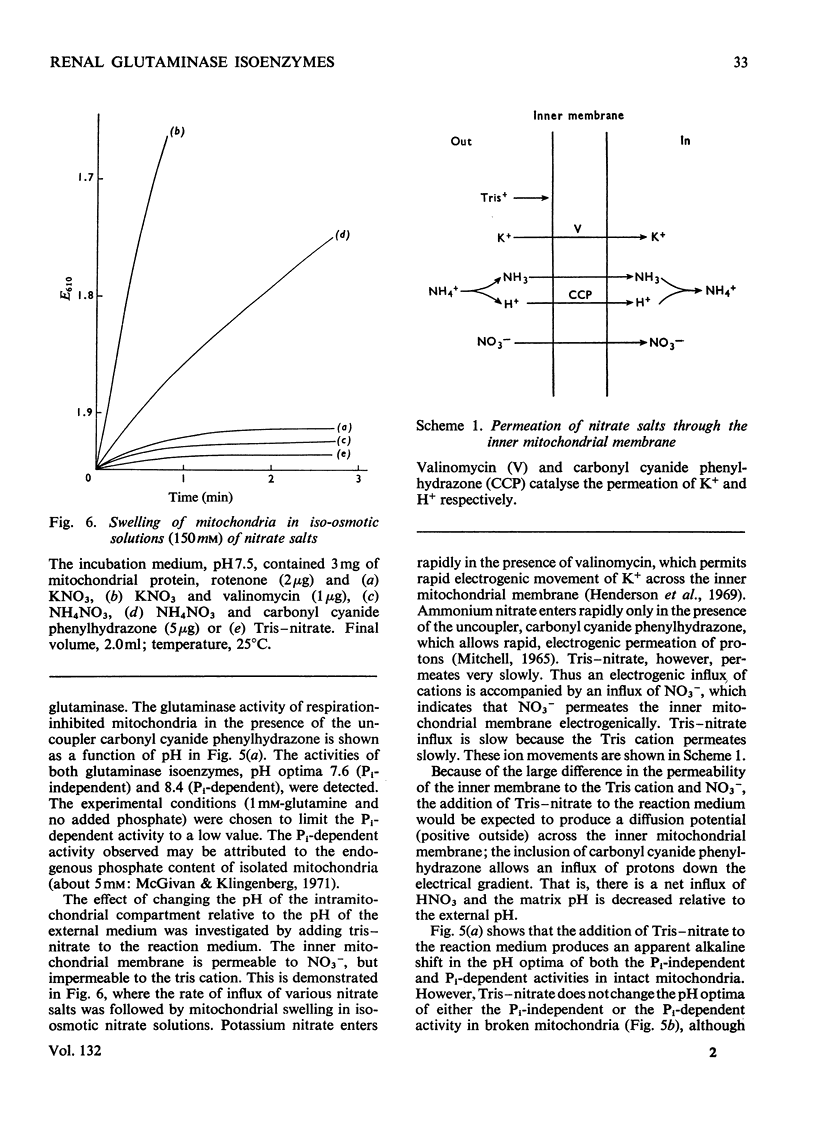
1. The glutaminase activity of pig kidney is located almost entirely in the cortex. 2. Pig renal cortex contains two glutaminases, one phosphate-dependent and one phosphate-independent. Both isoenzymes are localized exclusively in the mitochondria. 3. After sonication of the mitochondria, the phosphate-dependent isoenzyme is entirely soluble, whereas approximately half the phosphate-independent isoenzyme is associated with the membranes. 4. In intact mitochondria, the activities of both isoenzymes respond to changes in the pH of the intramitochondrial compartment. 5. It is concluded that both glutaminase isoenzymes are situated in the intramitochondrial compartment, and that the phosphate-independent glutaminase may be bound to the inside of the inner mitochondrial membrane.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chappell J. B. The oxidation of citrate, isocitrate and cis-aconitate by isolated mitochondria. Biochem J. 1964 Feb;90(2):225–237. doi: 10.1042/bj0900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M., Chappell J. B. Transport of glutamine and glutamate in kidney mitochondria in relation to glutamine deamidation. Biochem J. 1973 Jan;132(1):35–46. doi: 10.1042/bj1320035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUHA S. R. Intracellular localization of glutaminase I in rat liver. Enzymologia. 1961 May 15;23:94–100. [PubMed] [Google Scholar]
- Henderson P. J., McGivan J. D., Chappell J. B. The action of certain antibiotics on mitochondrial, erythrocyte and artificial phospholipid membranes. The role of induced proton permeability. Biochem J. 1969 Feb;111(4):521–535. doi: 10.1042/bj1110521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLINGMAN J. D., HANDLER P. Partial purification and properties of renal glutaminase. J Biol Chem. 1958 May;232(1):369–380. [PubMed] [Google Scholar]
- Katunuma N., Huzino A., Tomino I. Organ specific control of glutamine metabolism. Adv Enzyme Regul. 1967;5:55–69. doi: 10.1016/0065-2571(67)90008-8. [DOI] [PubMed] [Google Scholar]
- Katunuma N., Tomino I., Nishino H. Glutaminase isozymes in rat kidney. Biochem Biophys Res Commun. 1966 Feb 3;22(3):321–328. doi: 10.1016/0006-291x(66)90485-2. [DOI] [PubMed] [Google Scholar]
- LARDY H., COPENHAVER J. H., Jr Efficiency of oxidative phosphorylation. Nature. 1954 Jul 31;174(4422):231–232. doi: 10.1038/174231b0. [DOI] [PubMed] [Google Scholar]
- McGivan J. D., Klingenberg M. Correlation between H+ and anion movement in mitochondria and the key role of the phosphate carrier. Eur J Biochem. 1971 Jun 11;20(3):392–399. doi: 10.1111/j.1432-1033.1971.tb01405.x. [DOI] [PubMed] [Google Scholar]
- Rookledge K. A. Comparison of some metabolic parameters in the perfused and the incubated rat diaphragm muscle with diaphragm muscle in vivo. Biochem J. 1971 Nov;125(1):93–96. doi: 10.1042/bj1250093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAYRE F. W., ROBERTS E. Preparation and some properties of a phosphateactivated glutaminase from kidneys. J Biol Chem. 1958 Nov;233(5):1128–1134. [PubMed] [Google Scholar]


