Abstract
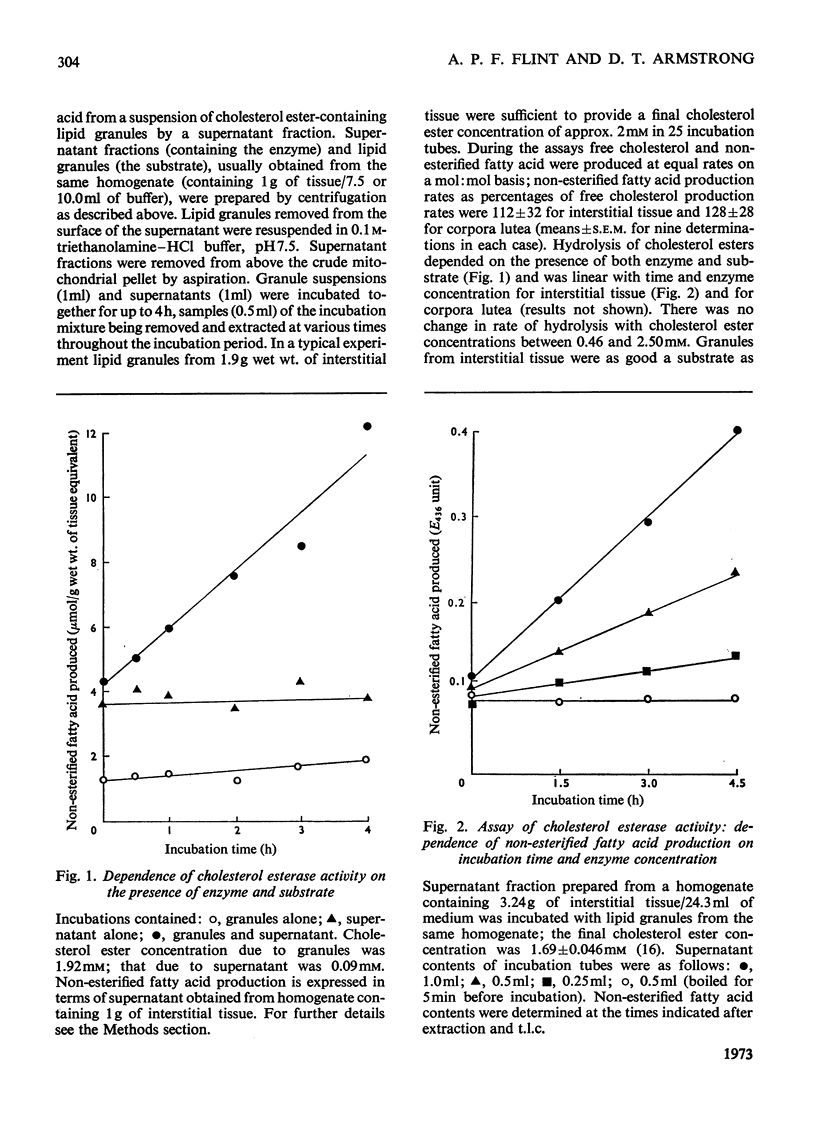
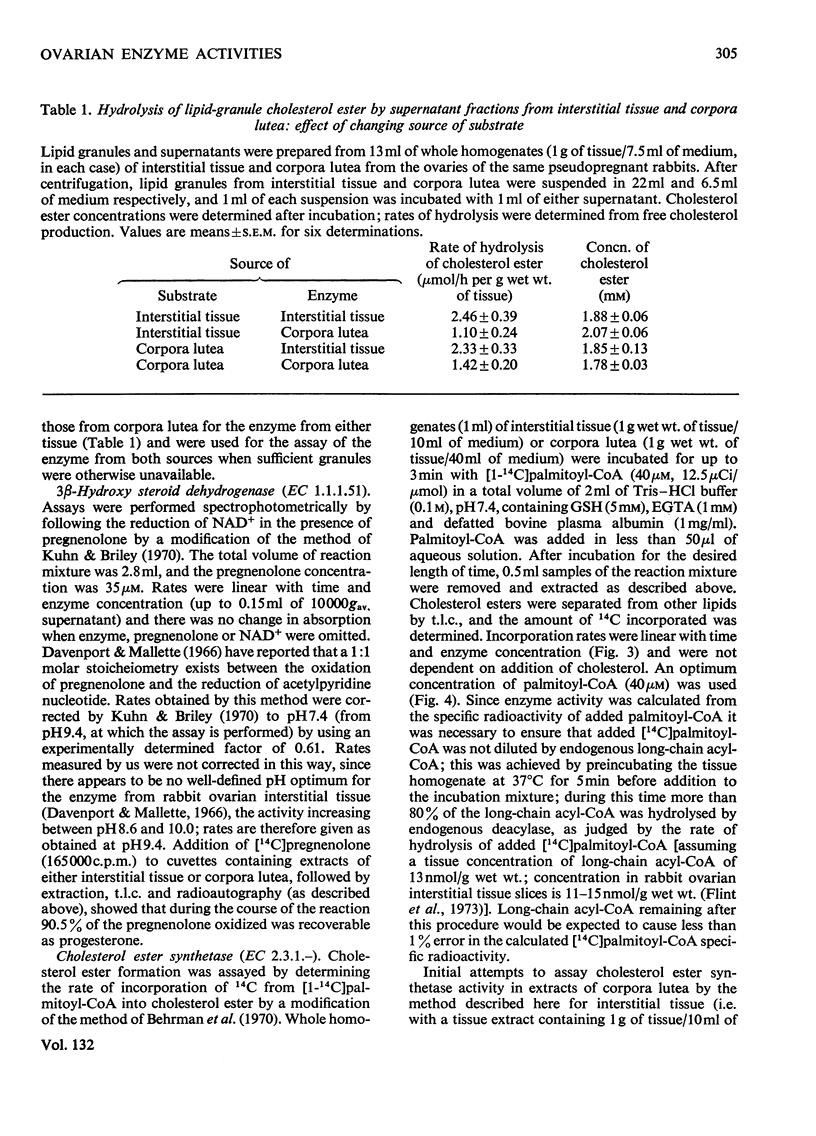
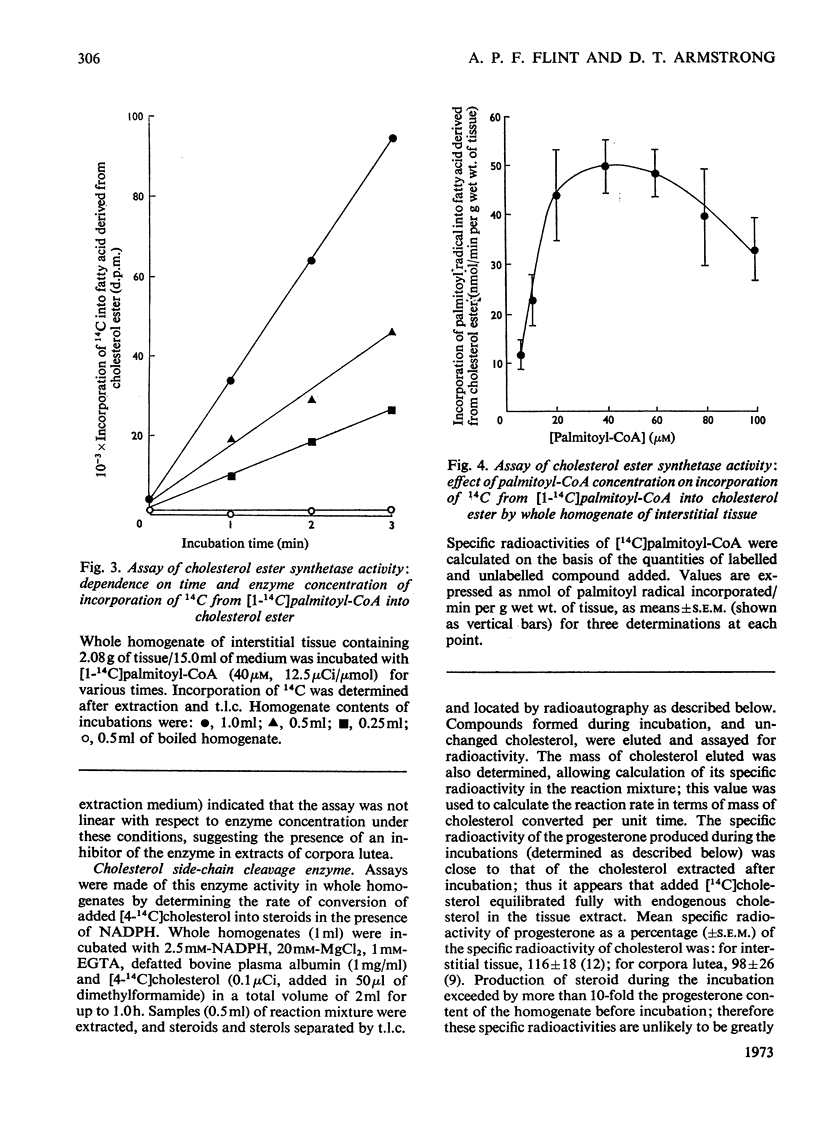
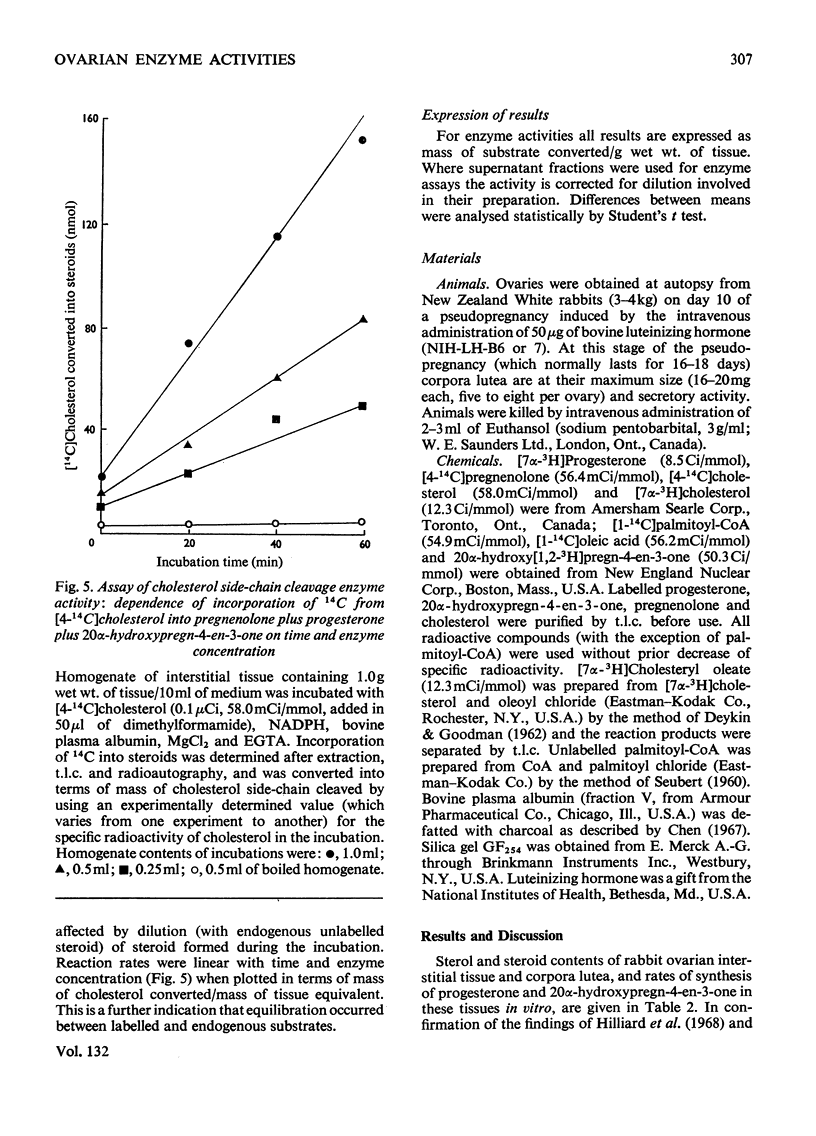
A method involving the use of isolated cholesterol ester-storage granules as substrate is described for the assay of cholesterol esterase in rabbit ovarian tissues. Activities of cholesterol esterase 100–200-fold higher than those previously reported in ovarian tissues were measured by using this method. In addition to that of cholesterol esterase, activities of cholesterol ester synthetase, cholesterol side-chain cleavage enzyme and 3β-hydroxy steroid dehydrogenase were determined in rabbit ovarian interstitial tissue and corpora lutea. Activities of these enzymes are in general compatible with the flows through them measured under a variety of conditions both in vivo and in vitro. It is concluded that, in the rabbit ovarian tissues investigated, these enzymes are capable of catalysing the conversions usually attributed to them.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. T. Gonadotropins, ovarian metabolism, and steroid biosynthesis. Recent Prog Horm Res. 1968;24:255–319. doi: 10.1016/b978-1-4831-9827-9.50012-5. [DOI] [PubMed] [Google Scholar]
- Behrman H. R., Amstrong D. T. Cholesterol esterase stimulation by luteinizing hormone in luteinized rat ovaries. Endocrinology. 1969 Sep;85(3):474–480. doi: 10.1210/endo-85-3-474. [DOI] [PubMed] [Google Scholar]
- Behrman H. R., Orczyk G. P., Macdonald G. J., Greep R. O. Prolactin induction of enzymes controlling luteal cholesterol ester turnover. Endocrinology. 1970 Dec;87(6):1251–1256. doi: 10.1210/endo-87-6-1251. [DOI] [PubMed] [Google Scholar]
- Bryson M. J., Sweat M. L. Cleavage of cholesterol side chain associated with cytochrome P-450, flavoprotein, and nonheme iron-protein derived from the bovine adrenal cortex. J Biol Chem. 1968 May 25;243(10):2799–2804. [PubMed] [Google Scholar]
- CLAESSON L., HILLARP N. A., HOGBERG B. Lipid changes in the interstitial gland of the rabbit ovary at oestrogen formation. Acta Physiol Scand. 1953 Nov 17;29(4):329–339. doi: 10.1111/j.1748-1716.1953.tb01028.x. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Coutts J. R., Stansfield D. A. Cholesteryl esterase and cholesteryl ester pools in corpus luteum. J Lipid Res. 1968 Sep;9(5):647–651. [PubMed] [Google Scholar]
- DEYKIN D., GOODMAN D. S. The hydrolysis of long-chain fatty acid esters of cholesterol with rat liver enzymes. J Biol Chem. 1962 Dec;237:3649–3656. [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- Davenport G. R., Mallette L. E. Some biochemical properties of rabbit ovarian hydroxysteroid dehydrogenases. Endocrinology. 1966 Apr;78(4):672–678. doi: 10.1210/endo-78-4-672. [DOI] [PubMed] [Google Scholar]
- Davies J., Davenport G. R., Norris J. L., Rennie P. I. Histochemical studies of hydroxysteroid dehydrogenase activity in mammalian reproductive tissues. Endocrinology. 1966 Apr;78(4):667–671. doi: 10.1210/endo-78-4-667. [DOI] [PubMed] [Google Scholar]
- Dorrington J. H., Kilpatrick R. Effects of pituitary hormones on progestational hormone production by the rabbit ovary in vivo and in vitro. J Endocrinol. 1966 May;35(1):53–63. doi: 10.1677/joe.0.0350053. [DOI] [PubMed] [Google Scholar]
- Duncombe W. G. The colorimetric micro-determination of long-chain fatty acids. Biochem J. 1963 Jul;88(1):7–10. doi: 10.1042/bj0880007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint A. P., Armstrong D. T. The compartmentation of non-esterified and esterified cholesterol in the superovulated rat ovary. Biochem J. 1971 Jun;123(2):143–152. doi: 10.1042/bj1230143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint A. P., Denton R. M. Metabolism of endogenous sterol ester by the superovulated rat ovary in vitro. Biochem J. 1970 Jan;116(1):79–82. doi: 10.1042/bj1160079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint A. P., Grinwich D. L., Armstrong D. T. Control of ovarian cholesterol ester biosynthesis. Biochem J. 1973 Feb;132(2):313–321. doi: 10.1042/bj1320313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN D. S., DEYKIN D., SHIRATORI T. THE FORMATION OF CHOLESTEROL ESTERS WITH RAT LIVER ENZYMES. J Biol Chem. 1964 May;239:1335–1345. [PubMed] [Google Scholar]
- Herbst A. L. Response of rat ovarian cholesterol to gonadotropins and anterior pituitary hormones. Endocrinology. 1967 Jul;81(1):54–60. doi: 10.1210/endo-81-1-54. [DOI] [PubMed] [Google Scholar]
- Hilliard J., Spies H. G., Sawyer C. H. Cholesterol storage and progestin secretion during pregnancy and pseudopregnancy in the rabbit. Endocrinology. 1968 Jan;82(1):157–165. doi: 10.1210/endo-82-1-157. [DOI] [PubMed] [Google Scholar]
- ITAYA K., UI M. COLORIMETRIC DETERMINATION OF FREE FATTY ACIDS IN BIOLOGICAL FLUIDS. J Lipid Res. 1965 Jan;6:16–20. [PubMed] [Google Scholar]
- Kuhn N. J., Briley M. S. The roles of pregn-5-ene-3 beta, 20 alpha-diol and 20 alpha-hydroxy steroid dehydrogenase in the control of progesterone synthesis preceding parturition and lactogenesis in the rat. Biochem J. 1970 Apr;117(2):193–201. doi: 10.1042/bj1170193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUKHERJEE S., KUNITAKE G., ALFINSLATER R. B. The esterification of cholesterol with palmitic acid by rat liver homogenates. J Biol Chem. 1958 Jan;230(1):91–96. [PubMed] [Google Scholar]
- Morin R. J., Eras J., Martin S. C. Hydrolysis of cholesteryl oleate in vitro by ovarian homogenates from non-pregnant and pregnant rabbits. Life Sci. 1969 Sep 15;8(18):995–999. doi: 10.1016/0024-3205(69)90206-9. [DOI] [PubMed] [Google Scholar]
- RUBIN B. L., DEANE H. W., HAMILTON J. A. BIOCHEMICAL AND HISTOCHEMICAL STUDIES ON 3-BETA-HYDROXYSTEROID DEHYDROGENASE ACTIVITY IN THE ADRENAL GLANDS AND OVARIES OF DIVERSE MAMMALS. Endocrinology. 1963 Dec;73:748–763. doi: 10.1210/endo-73-6-748. [DOI] [PubMed] [Google Scholar]
- STORMSHAK F., CASIDA L. E. EFFECT OF GONADOTROPINS ON CORPORA LUTEA OF PSEUDOPREGNANT RABBITS. Endocrinology. 1964 Sep;75:321–325. doi: 10.1210/endo-75-3-321. [DOI] [PubMed] [Google Scholar]
- Simpson E. R., Boyd G. S. Partial resolution of the mixed-function oxidase involved in the cholesterol side-chain cleavage reaction in bovine adrenal mitochondria. Biochem Biophys Res Commun. 1967 Sep 27;28(6):945–950. doi: 10.1016/0006-291x(67)90071-x. [DOI] [PubMed] [Google Scholar]
- Solod E. A., Armstrong D. T., Greep R. O. Action of luteinizing hormone on conversion of ovarian cholesterol stores to steroids secreted in vivo and synthesized in vitro by the pseudopregnant rabbit ovary. Steroids. 1966 Jun;7(6):607–620. doi: 10.1016/0039-128x(66)90147-4. [DOI] [PubMed] [Google Scholar]
- Strauss J. F., 3rd, Foley B., Stambaugh R. 20 -hydroxysteroid dehydrogenase activity in the rabbit ovary. Biol Reprod. 1972 Feb;6(1):78–86. doi: 10.1093/biolreprod/6.1.78. [DOI] [PubMed] [Google Scholar]
- Sulimovici S., Boyd G. S. The effect of ascorbic acid in vitro on the rat ovarian cholesterol side chain cleavage enzyme system. Steroids. 1968 Jul;12(1):127–149. doi: 10.1016/s0039-128x(68)80085-6. [DOI] [PubMed] [Google Scholar]
- Wilks J. W., Fuller G. B., Hansel W. The role of cholesterol as a progestin precursor in rat, rabbit, and bovine luteal tissue. Endocrinology. 1970 Sep;87(3):581–587. doi: 10.1210/endo-87-3-581. [DOI] [PubMed] [Google Scholar]


