Abstract
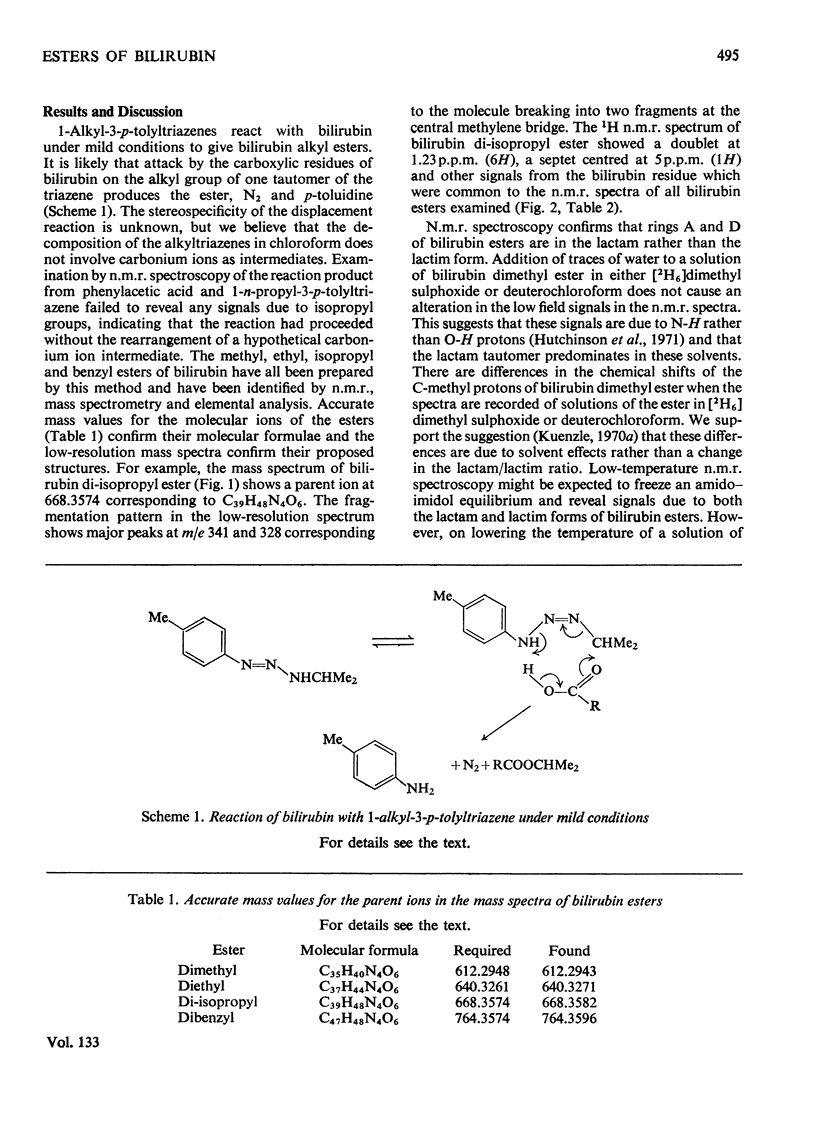
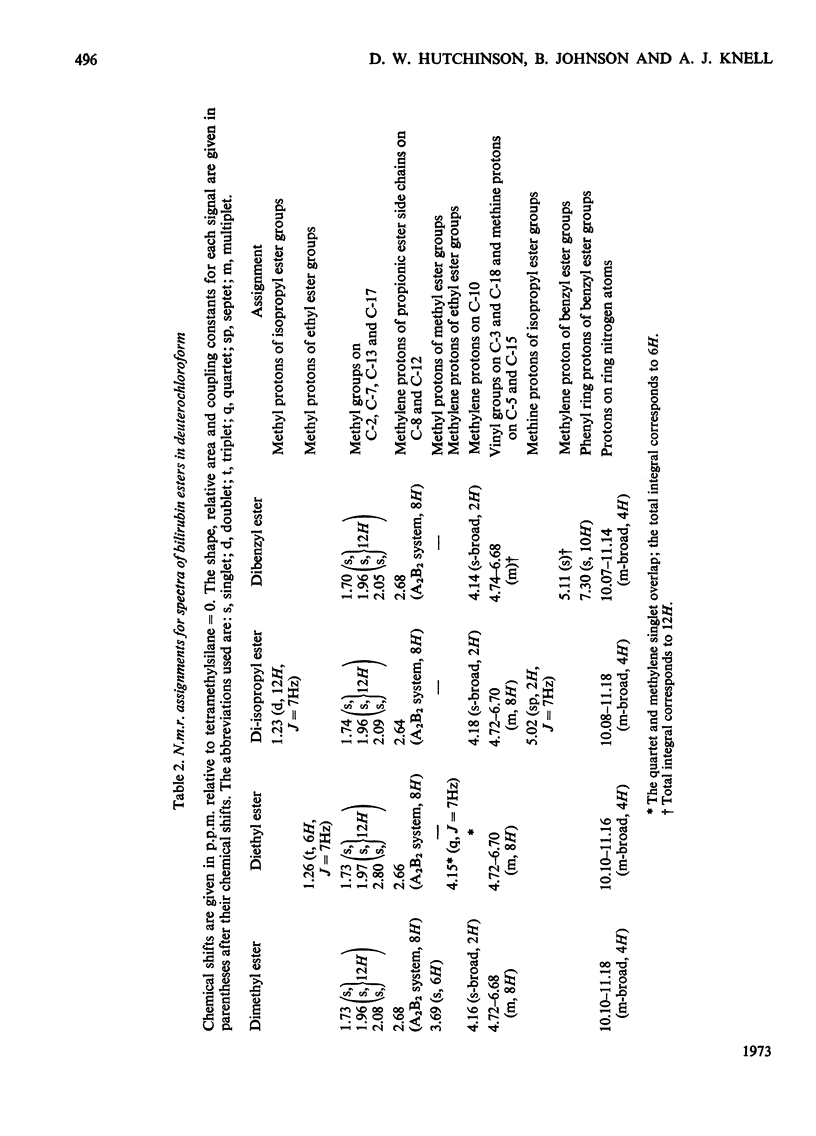
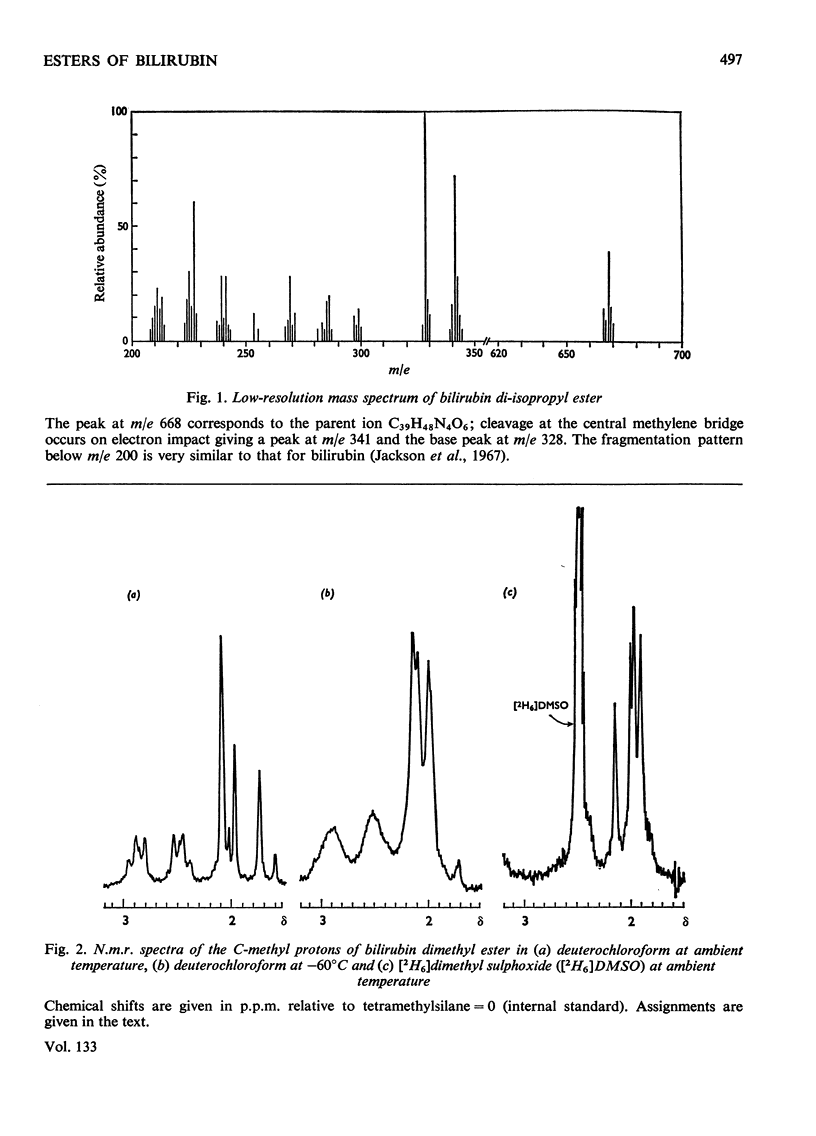
1-Alkyl-3-p-tolytriazenes were used to synthesize the methyl, ethyl, isopropyl and benzyl esters of bilirubin. Treatment of a chloroform solution of bilirubin with the triazene at room temperature gave high yields of the corresponding esters. These were identified by n.m.r. and mass spectroscopy together with elemental analysis. N.m.r. studies also suggest that bilirubin dimethyl ester is in the lactam rather than the lactim form.
Full text
PDF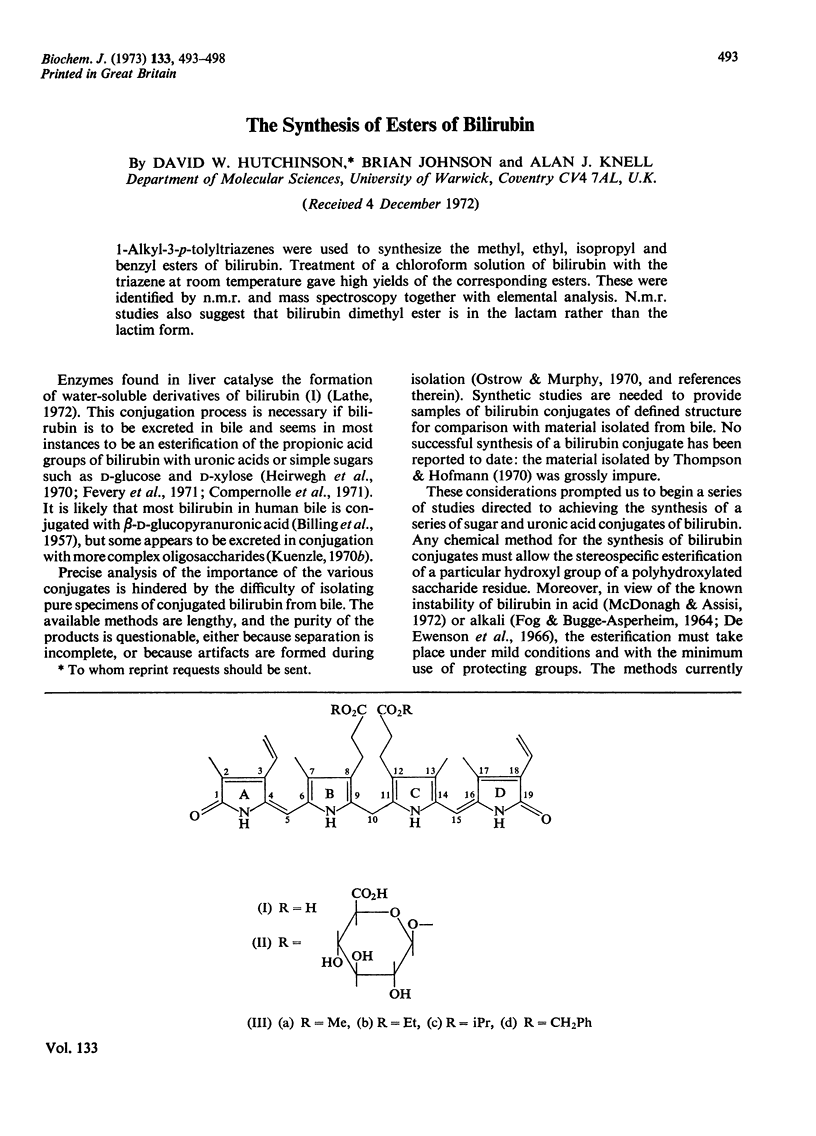


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILLING B. H., COLE P. G., LATHE G. H. The excretion of bilirubin as a diglucuronide giving the direct van den Bergh reaction. Biochem J. 1957 Apr;65(4):774–784. doi: 10.1042/bj0650774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compernolle F., Van Hees G. P., Fevery J., Heirwegh K. P. Mass-spectrometric structure elucidation of dog bile azopigments as the acyl glycosides of glucopyranose and xylopyranose. Biochem J. 1971 Dec;125(3):811–819. doi: 10.1042/bj1250811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ewenson I. W., Gianturco F. A., Gramaccioni P. On the stability of bilirubin. Experientia. 1966 Jan 15;22(1):14–15. doi: 10.1007/BF01897739. [DOI] [PubMed] [Google Scholar]
- FOG J. BILIRUBIN-PURIFICATION-PURITY. Scand J Clin Lab Invest. 1964;16:49–54. doi: 10.3109/00365516409060482. [DOI] [PubMed] [Google Scholar]
- FOG J., BUGGE-ASPERHEIM B. STABILITY OF BILIRUBIN. Nature. 1964 Aug 15;203:756–757. doi: 10.1038/203756a0. [DOI] [PubMed] [Google Scholar]
- Fevery J., Van Hees G. P., Leroy P., Compernolle F., Heirwegh K. P. Excretion in dog bile of glucose and xylose conjugates of bilirubin. Biochem J. 1971 Dec;125(3):803–810. doi: 10.1042/bj1250803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson D. W., Johnson B., Knell A. J. Tautomerism and hydrogen bonding in bilirubin. Biochem J. 1971 Jul;123(3):483–484. doi: 10.1042/bj1230483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. Nuclear-magnetic-resonance, infrared and optical spectra of model compounds. Biochem J. 1970 Sep;119(3):395–409. doi: 10.1042/bj1190395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. The excretion of bilirubin as the acyl glycosides of aldobiouronic acid, pseudoaldobiouronic acid and hexuronosylhexuronic acid, with a branched-chain hexuronic acid as one of the components of the hexuronosylhexuronide. Biochem J. 1970 Sep;119(3):411–435. doi: 10.1042/bj1190411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe G. H. The degradation of haem by mammals and its excretion as conjugated bilirubin. Essays Biochem. 1972;8:107–148. [PubMed] [Google Scholar]
- Nichol A. W., Morell D. B. Tautomerism and hydrogen bonding in bilirubin and biliverdin. Biochim Biophys Acta. 1969 May 6;177(3):599–609. doi: 10.1016/0304-4165(69)90325-0. [DOI] [PubMed] [Google Scholar]
- Ostrow J. D., Murphy N. H. Isolation and properties of conjugated bilirubin from bile. Biochem J. 1970 Nov;120(2):311–327. doi: 10.1042/bj1200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryka Z. J., Watson C. J. Separation of bile pigments by thin layer chromatography. J Chromatogr. 1968 Sep 24;37(1):76–82. doi: 10.1016/s0021-9673(01)99073-9. [DOI] [PubMed] [Google Scholar]


