Abstract
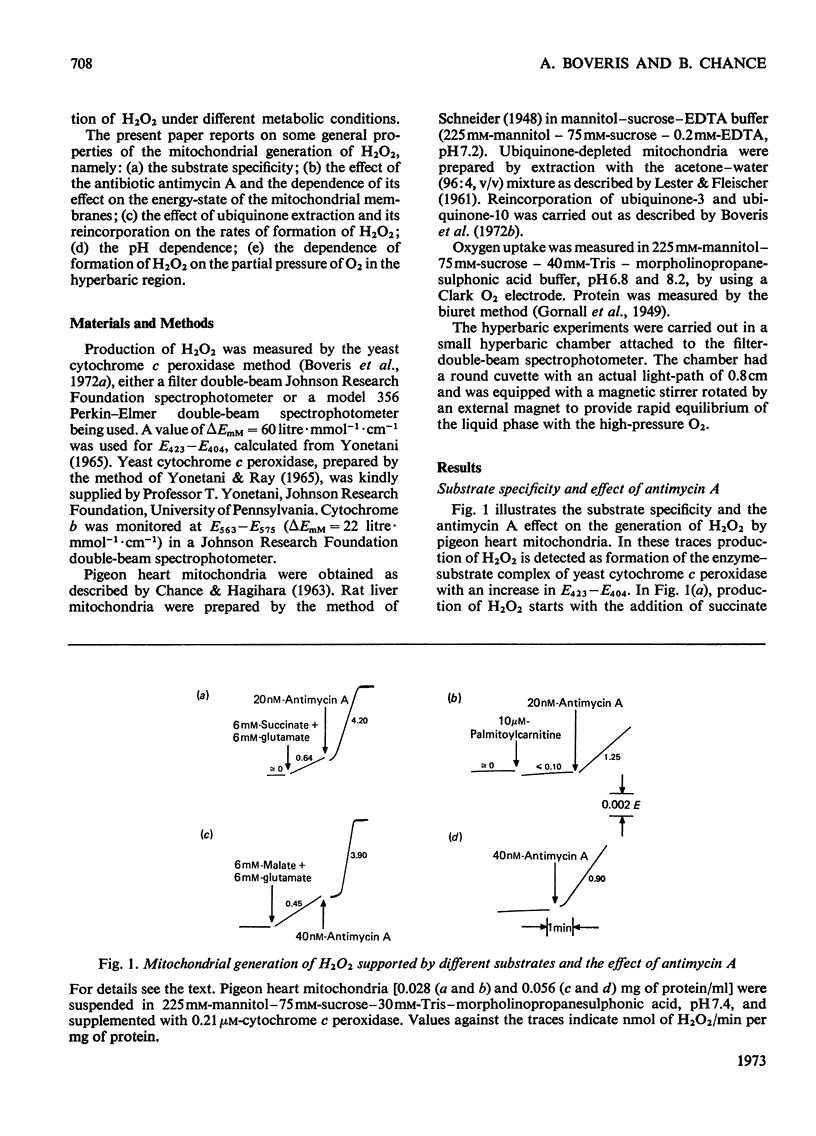
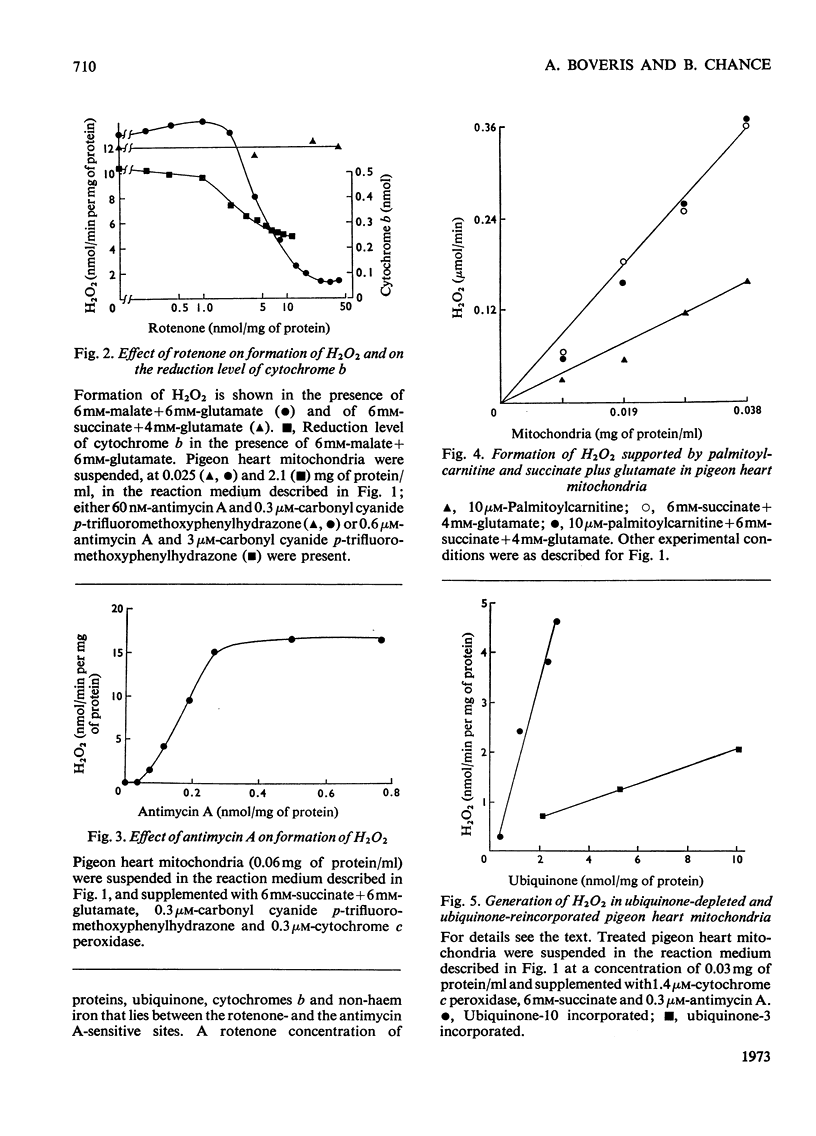
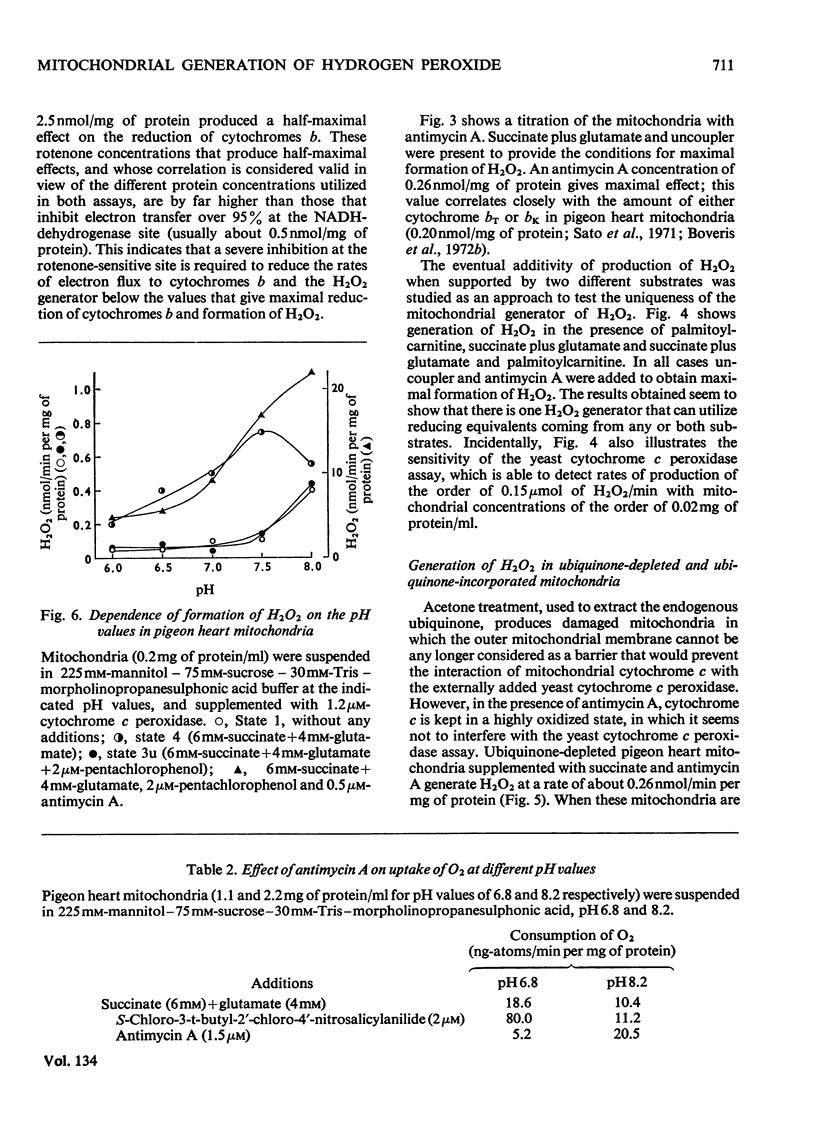
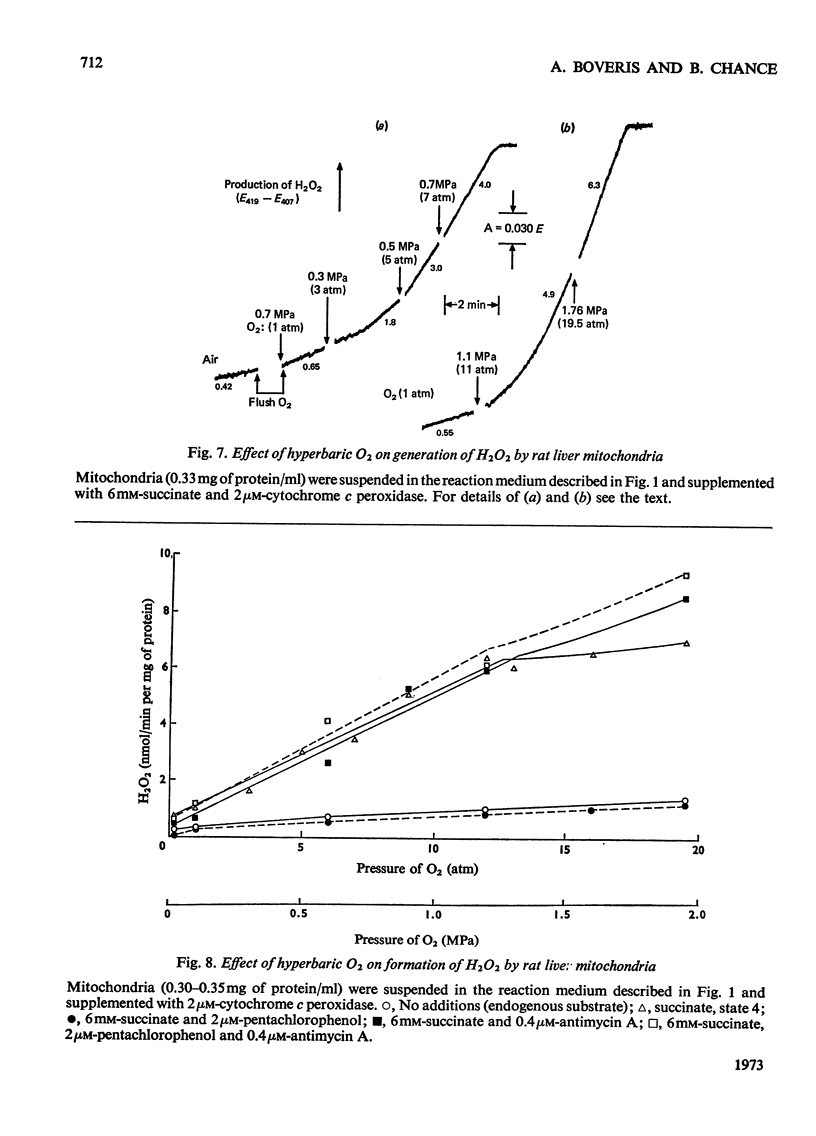
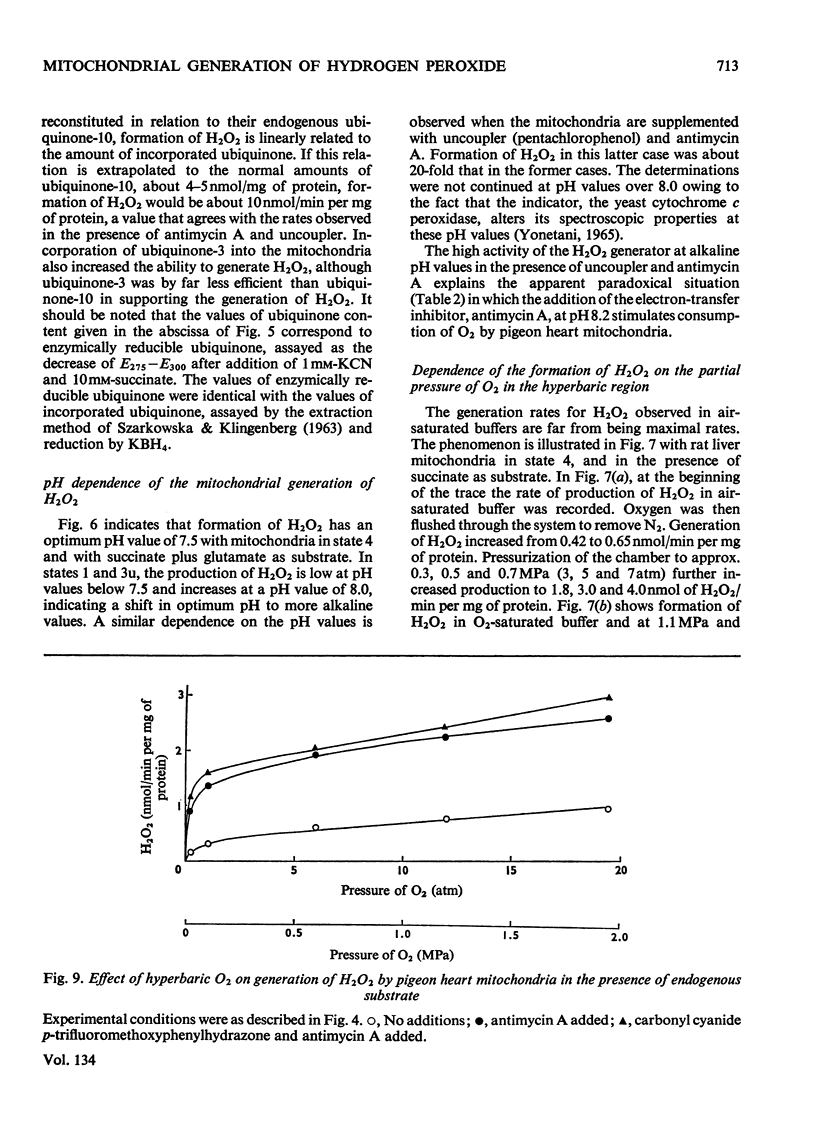
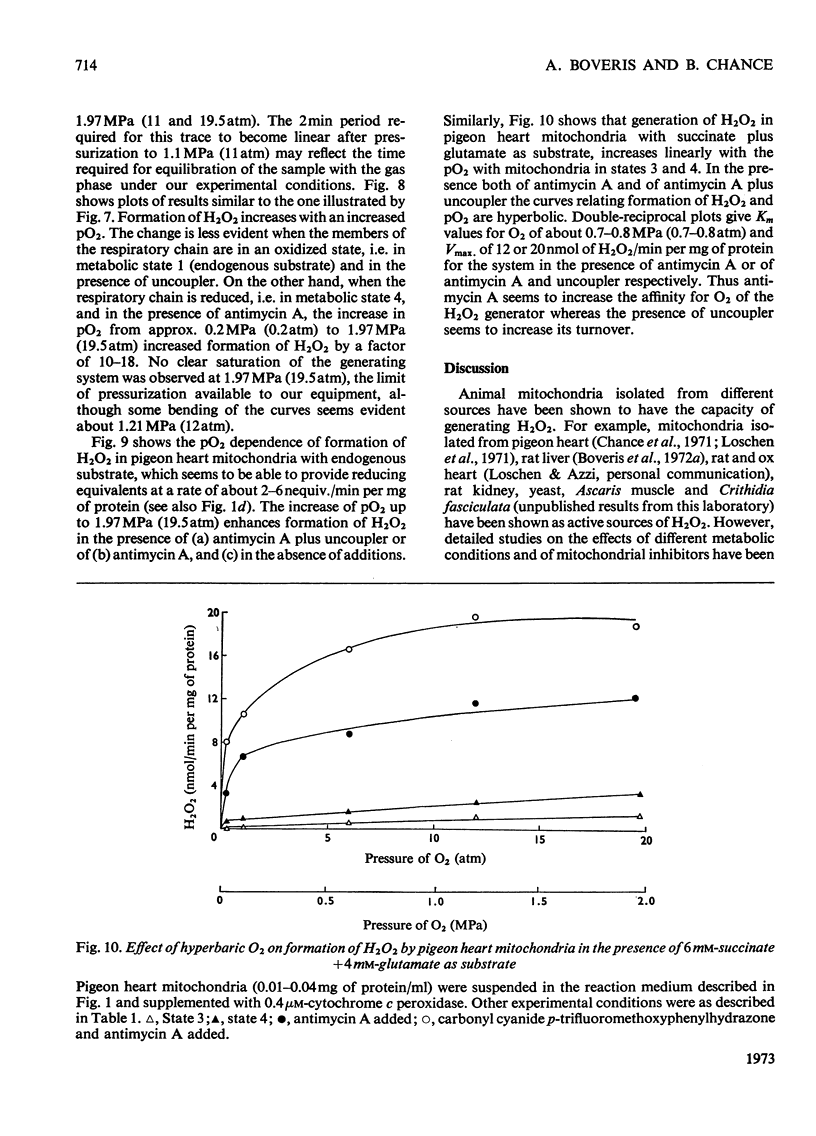
1. Pigeon heart mitochondria produce H2O2 at a maximal rate of about 20nmol/min per mg of protein. 2. Succinate–glutamate and malate–glutamate are substrates which are able to support maximal H2O2 production rates. With malate–glutamate, H2O2 formation is sensitive to rotenone. Endogenous substrate, octanoate, stearoyl-CoA and palmitoyl-carnitine are by far less efficient substrates. 3. Antimycin A exerts a very pronounced effect in enhancing H2O2 production in pigeon heart mitochondria; 0.26nmol of antimycin A/mg of protein and the addition of an uncoupler are required for maximal H2O2 formation. 4. In the presence of endogenous substrate and of antimycin A, ATP decreases and uncoupler restores the rates of H2O2 formation. 5. Reincorporation of ubiquinone-10 and ubiquinone-3 to ubiquinone-depleted pigeon heart mitochondria gives a system in which H2O2 production is linearly related to the incorporated ubiquinone. 6. The generation of H2O2 by pigeon heart mitochondria in the presence of succinate–glutamate and in metabolic state 4 has an optimum pH value of 7.5. In states 1 and 3u, and in the presence of antimycin A and uncoupler, the optimum pH value is shifted towards more alkaline values. 7. With increase of the partial pressure of O2 to the hyperbaric region the formation of H2O2 is markedly increased in pigeon heart mitochondria and in rat liver mitochondria. With rat liver mitochondria and succinate as substrate in state 4, an increase in the pO2 up to 1.97MPa (19.5atm) increases H2O2 formation 10–15-fold. Similar pO2 profiles were observed when rat liver mitochondria were supplemented either with antimycin A or with antimycin A and uncoupler. No saturation of the system with O2 was observed up to 1.97MPa (19.5atm). By increasing the pO2 to 1.97MPa (19.5atm), H2O2 formation in pigeon heart mitochondria with succinate as substrate increased fourfold in metabolic state 4, with antimycin A added the increase was threefold and with antimycin A and uncoupler it was 2.5-fold. In the last two saturation of the system with oxygen was observed, with an apparent Km of about 71kPa (0.7–0.8atm) and a Vmax. of 12 and 20nmol of H2O2/min per mg of protein. 8. It is postulated that in addition to the well-known flavin reaction, formation of H2O2 may be due to interaction with an energy-dependent component of the respiratory chain at the cytochrome b level.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREAE W. A. A sensitive method for the estimation of hydrogen peroxide in biological materials. Nature. 1955 May 14;175(4463):859–860. doi: 10.1038/175859a0. [DOI] [PubMed] [Google Scholar]
- BEINERT H., HEINEN W., PALMER G. Applications of combined low temperature optical and electron paramagnetic resonance spectroscopy to the study of oxidative enzymes. Brookhaven Symp Biol. 1962 Dec;15:229–265. [PubMed] [Google Scholar]
- Boveris A., Erecińska M., Wagner M. Reduction kinetics of cytochromes b. Biochim Biophys Acta. 1972 Feb 28;256(2):223–242. doi: 10.1016/0005-2728(72)90055-2. [DOI] [PubMed] [Google Scholar]
- Boveris A., Oshino N., Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972 Jul;128(3):617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Chance B., Jamieson D., Coles H. Energy-linked pyridine nucleotide reduction: inhibitory effects of hyperbaric oxygen in vitro and in vivo. Nature. 1965 Apr 17;206(981):257–263. doi: 10.1038/206257a0. [DOI] [PubMed] [Google Scholar]
- Chance B., Oshino N. Kinetics and mechanisms of catalase in peroxisomes of the mitochondrial fraction. Biochem J. 1971 Apr;122(2):225–233. doi: 10.1042/bj1220225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernster L., Lee I. -Y., Norling B., Persson B. Studies with ubiquinone-depleted submitochondrial particles. FEBS Lett. 1969 Apr;3(1):21–26. doi: 10.1016/0014-5793(69)80086-4. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Chance B., Ernster L., Lee C. P., Wong D. Flavoproteins of mitochondrial fatty acid oxidation. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1696–1702. doi: 10.1073/pnas.58.4.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman M., Kearney E. B., Singer T. P. Control of succinate dehydrogenase in mitochondria. Biochemistry. 1971 Dec 7;10(25):4763–4770. doi: 10.1021/bi00801a025. [DOI] [PubMed] [Google Scholar]
- Hinkle P. C., Butow R. A., Racker E., Chance B. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XV. Reverse electron transfer in the flavin-cytochrome beta region of the respiratory chain of beef heart submitochondrial particles. J Biol Chem. 1967 Nov 25;242(22):5169–5173. [PubMed] [Google Scholar]
- Jensen P. K. Antimycin-insensitive oxidation of succinate and reduced nicotinamide-adenine dinucleotide in electron-transport particles. I. pH dependency and hydrogen peroxide formation. Biochim Biophys Acta. 1966 Aug 10;122(2):157–166. doi: 10.1016/0926-6593(66)90057-9. [DOI] [PubMed] [Google Scholar]
- Jensen P. K. Antimycin-insensitive oxidation of succinate and reduced nicotinamide-adenine dinucleotide in electron-transport particles. II. Steroid effects. Biochim Biophys Acta. 1966 Aug 10;122(2):167–174. doi: 10.1016/0926-6593(66)90058-0. [DOI] [PubMed] [Google Scholar]
- LESTER R. L., FLEISCHER S. Studies on the electron-transport system. 27. The respiratory activity of acetoneextracted beef-heart mitochondria: role of coenzyme Q and other lipids. Biochim Biophys Acta. 1961 Feb 18;47:358–377. doi: 10.1016/0006-3002(61)90297-9. [DOI] [PubMed] [Google Scholar]
- Loschen G., Flohé L., Chance B. Respiratory chain linked H(2)O(2) production in pigeon heart mitochondria. FEBS Lett. 1971 Nov 1;18(2):261–264. doi: 10.1016/0014-5793(71)80459-3. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The generation of superoixide radical during the autoxidation of ferredoxins. J Biol Chem. 1971 Nov 25;246(22):6886–6890. [PubMed] [Google Scholar]
- Oshino N., Chance B., Sies H., Bücher T. The role of H 2 O 2 generation in perfused rat liver and the reaction of catalase compound I and hydrogen donors. Arch Biochem Biophys. 1973 Jan;154(1):117–131. doi: 10.1016/0003-9861(73)90040-4. [DOI] [PubMed] [Google Scholar]
- Rossi E., Norling B., Persson B., Ernster L. Studies with ubiquinone-depleted submitochondrial particles. Effects of extraction and reincorporation of ubiquinone on the kinetics of succinate dehydrogenase. Eur J Biochem. 1970 Nov;16(3):508–513. doi: 10.1111/j.1432-1033.1970.tb01110.x. [DOI] [PubMed] [Google Scholar]
- SZARKOWSKA L., KLINGENBERG M. ON THE ROLE OF UBIQUINONE IN MITOCHONDRIA. SPECTROPHOTOMETRIC AND CHEMICAL MEASUREMENTS OF ITS REDOX REACTIONS. Biochem Z. 1963;338:674–697. [PubMed] [Google Scholar]
- Sato N., Wilson D. F., Chance B. The spectral properties of the b cytochromes in intact mitochondria. Biochim Biophys Acta. 1971 Nov 2;253(1):88–97. doi: 10.1016/0005-2728(71)90236-2. [DOI] [PubMed] [Google Scholar]
- Sies H., Chance B. The steady state level of catalase compound I in isolated hemoglobin-free perfused rat liver. FEBS Lett. 1970 Dec;11(3):172–176. doi: 10.1016/0014-5793(70)80521-x. [DOI] [PubMed] [Google Scholar]
- Yonetani T., Ray G. S. Studies on cytochrome c peroxidase. I. Purification and some properties. J Biol Chem. 1965 Nov;240(11):4503–4508. [PubMed] [Google Scholar]
- Yonetani T. Studies on cytochrome c peroxidase. II. Stoichiometry between enzyme, H2O2, and ferrocytochrome c and enzymic determination of extinction coefficients of cytochrome c. J Biol Chem. 1965 Nov;240(11):4509–4514. [PubMed] [Google Scholar]


