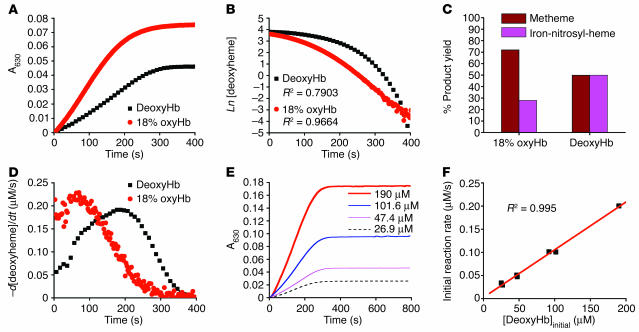
Figure 1.
Reaction of nitrite with deoxyHb reveals deviation from first-order kinetics and equimolar product yields of metHb and iron-nitrosyl-Hb. (A) Progress of the nitrite-Hb reaction under conditions of excess nitrite (10 mM nitrite with 50 μM heme) monitored spectrophotometrically by formation of ferric hemes at 630 nm (baseline at 700 nm) under anaerobic conditions or during partial oxygenation (18% oxyHb). 50 μM metHb gives A630 of 0.12 with 10 mM nitrite present. (B) The fit of the natural log of deoxyheme concentration versus time for conditions shown in A, where Ln[deoxyheme] is the natural log of the deoxyheme concentration at each time point. The deoxyheme concentration at each time point was determined by spectral deconvolution. (C) Effects of oxygen leak on product yields in the nitrite-Hb reaction (calculated by spectral deconvolution for reactions in A). (D) Instantaneous rate of deoxyheme consumption over time during the course of a single reaction under the conditions in A. The instantaneous rate of deoxyheme consumption was found by negative change of deoxyheme concentration between 2 adjacent time points (–d[deoxyheme]) over the time interval (dt) where d is change and t is time. (E) Progress of the reaction of nitrite (10 mM) with varying initial concentrations of deoxyheme monitored by the formation of metheme at 630 nm. (F) Effect of varying the initial deoxyheme concentration (concentration of deoxyheme at the beginning of the reaction; [DeoxyHb]initial) on the initial rate of the reaction calculated from the data in E. The initial reaction rate is the rate of ferric heme formation at the beginning of the reaction calculated as the average rate over the first 200 seconds using extinction coefficient of 3.4 at 630 nm (the rate of deoxyheme consumption is approximately twice as fast).