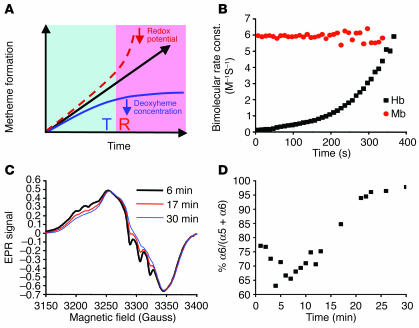
Figure 2.
Sigmoidal reaction behavior of the nitrite-deoxyheme reaction occurs due to T-to-R allosteric quaternary transition of Hb. (A) Model of the nitrite-deoxyHb reaction representing a balance between 2 opposing processes: (a) a decelerating reaction with T state deoxyheme due to depletion of deoxyheme available for reaction with nitrite because of conversion to metheme and iron-nitrosyl-heme (solid blue line); and (b) an accelerating reaction of nitrite with R state deoxyheme (dashed line). Solid black line with arrow represents the reaction process observed experimentally and is the balance of these 2 processes. (B) Apparent bimolecular rate constant over the time course of the anaerobic reaction of Mb (50 μM heme) and Hb (50 μM heme) with nitrite (10 mM with Hb and 2.5 mM with Mb in heme concentrations). Bimolecular rate constant (const.) was obtained by dividing the instantaneous reaction rate of deoxyheme consumption by the concentration of deoxyheme and nitrite. (C) Initial (6 minutes), intermediate (17 minutes), and final (30 minutes) EPR spectra of iron-nitrosyl-heme monitored over the course of the nitrite-deoxyHb reaction (100 μM heme, 2.5 mM nitrite) showing a transition from 5-coordinate (T state) α iron-nitrosyl-heme (with characteristic hyperfine splitting) to 6-coordinate (R state) heme geometry. The smaller EPR signals at earlier time points were normalized to that of the final time point in order to compare the spectral shape of the iron-nitrosyl-Hb signal. (D) T-to-R allosteric structural transition during the course of the nitrite-deoxyHb reaction, monitored by the percentage formation of 6-coordinate α iron-nitrosyl-heme relative to total α iron-nitrosyl-heme.