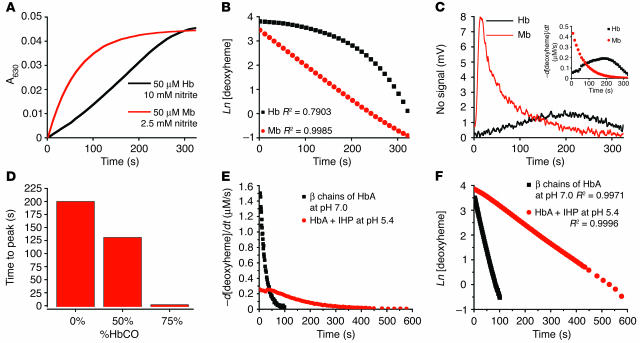
Figure 3.
Rate of nitrite reductase reaction and NO gas formation is under allosteric control. (A) Progress of the anaerobic reaction of Mb (50 μM heme) and Hb (50 μM heme) with nitrite (10 mM with Hb and 2.5 mM with Mb in heme concentrations) monitored spectrophotometrically by metHb formation at 630 nm. (B) First order fits for Mb and non–first order behavior of tetrameric Hb (fits of natural log of deoxyheme concentration for the same reactions shown in A). (C) Simultaneous measurement of NO gas by chemiluminescence during the course of the reaction shown in A. Inset shows the instantaneous rate of deoxyheme consumption over the course of the reaction, obtained from spectral deconvolution. (D) The time to peak NO production measured by chemiluminescence for the reaction of nitrite (10 mM) with Hb (50 μM heme) with varying saturation (0–75%) of carbon monoxide. %HbCO, percentage of Hb that is saturated with carbon monoxide. (E) Reaction progress for β chains of HbA (locked in R state tetramer; 35 μM heme reacted with 0.5 mM nitrite at pH 7.0) and IHP-treated Hb (locked into T state tetramer; 50 μM heme reacted with 2.5 mM nitrite at pH 6.4) was monitored by the rate of deoxyheme consumption. (F) First-order fits for β chains of HbA at pH 7.0 and for IHP-treated Hb at pH 6.4 for conditions in E showing that the deviation from first order requires an allosteric structural transition of the Hb tetramer (fits of natural log of deoxyheme concentration for the same reactions shown in E).