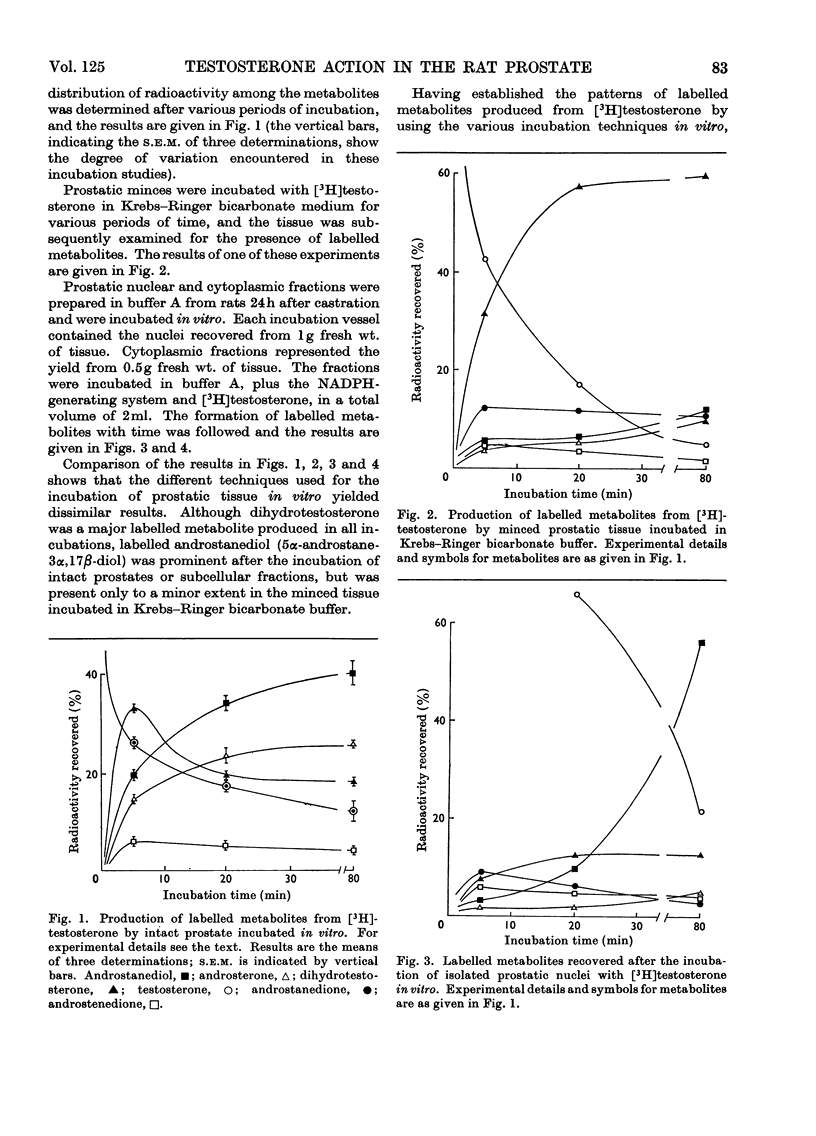
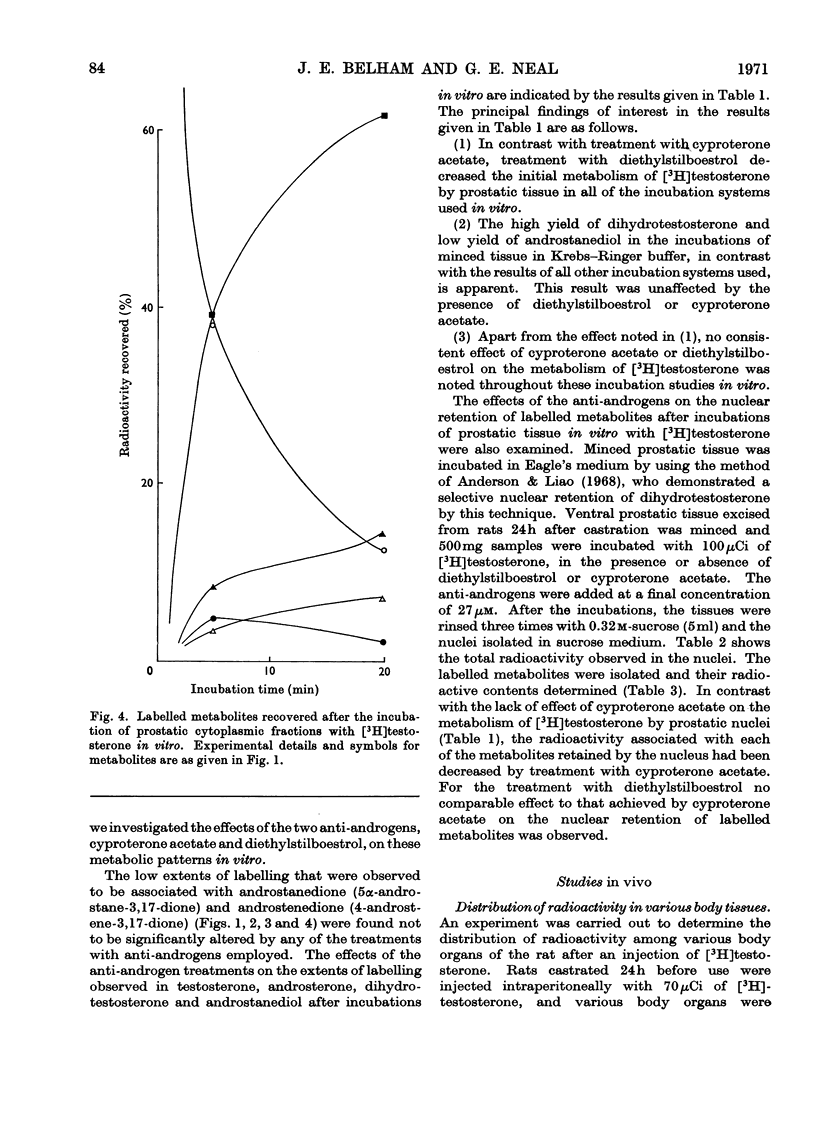
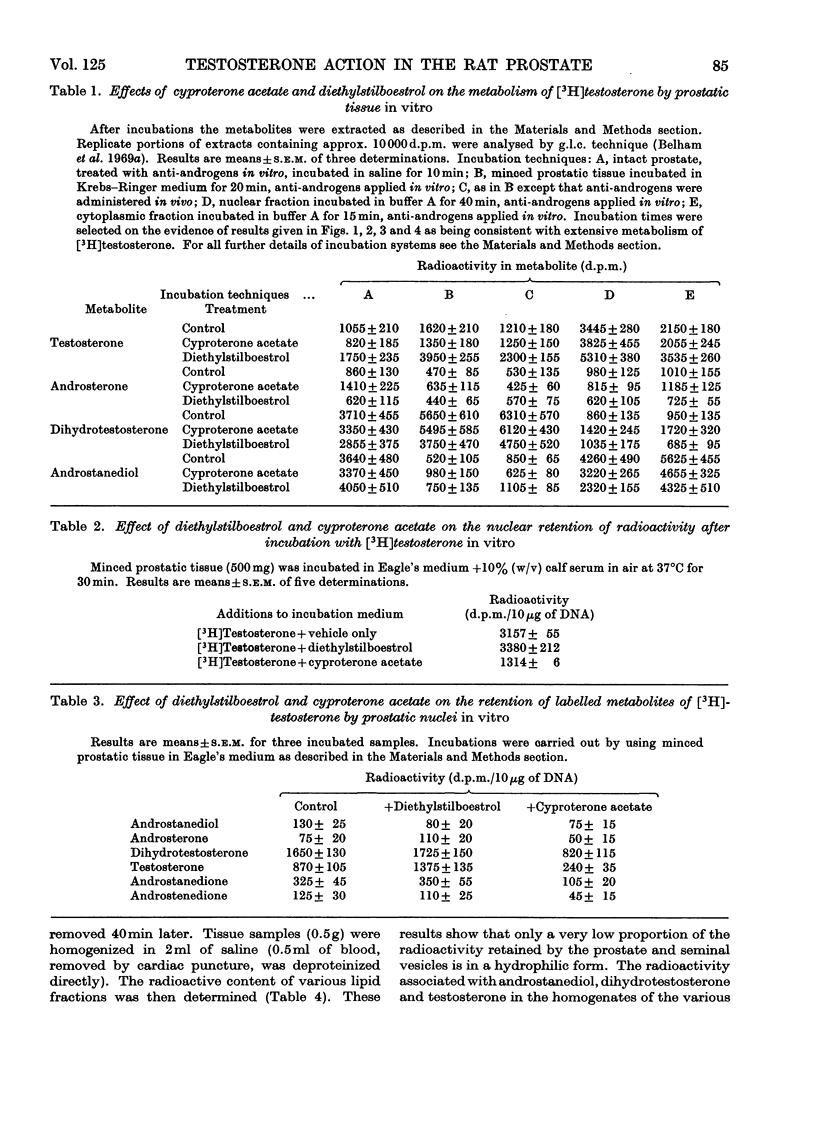
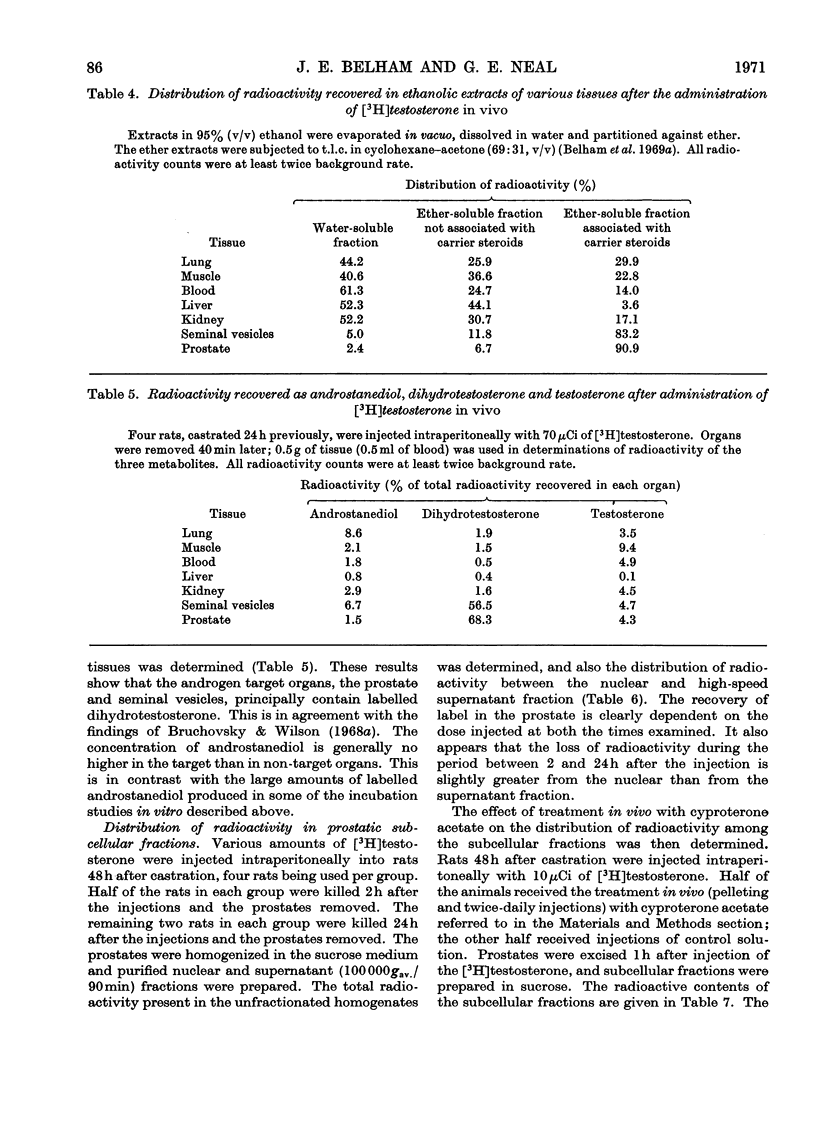
Abstract
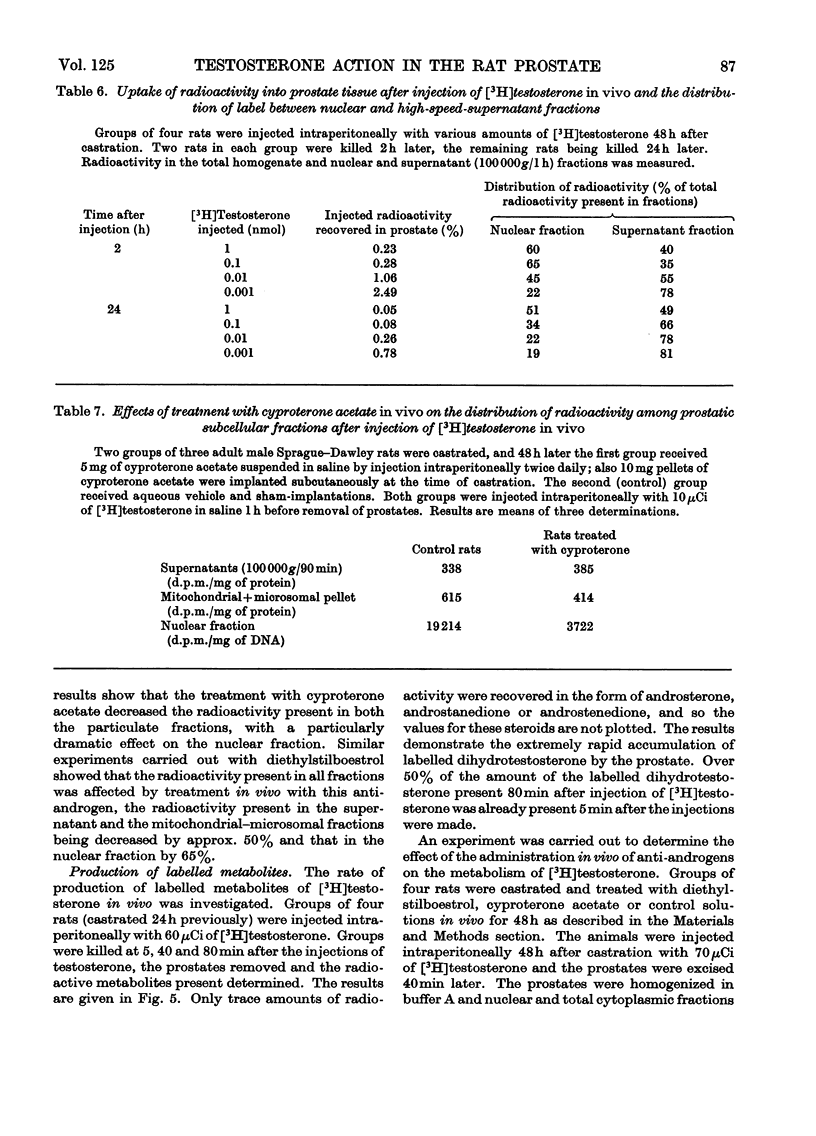
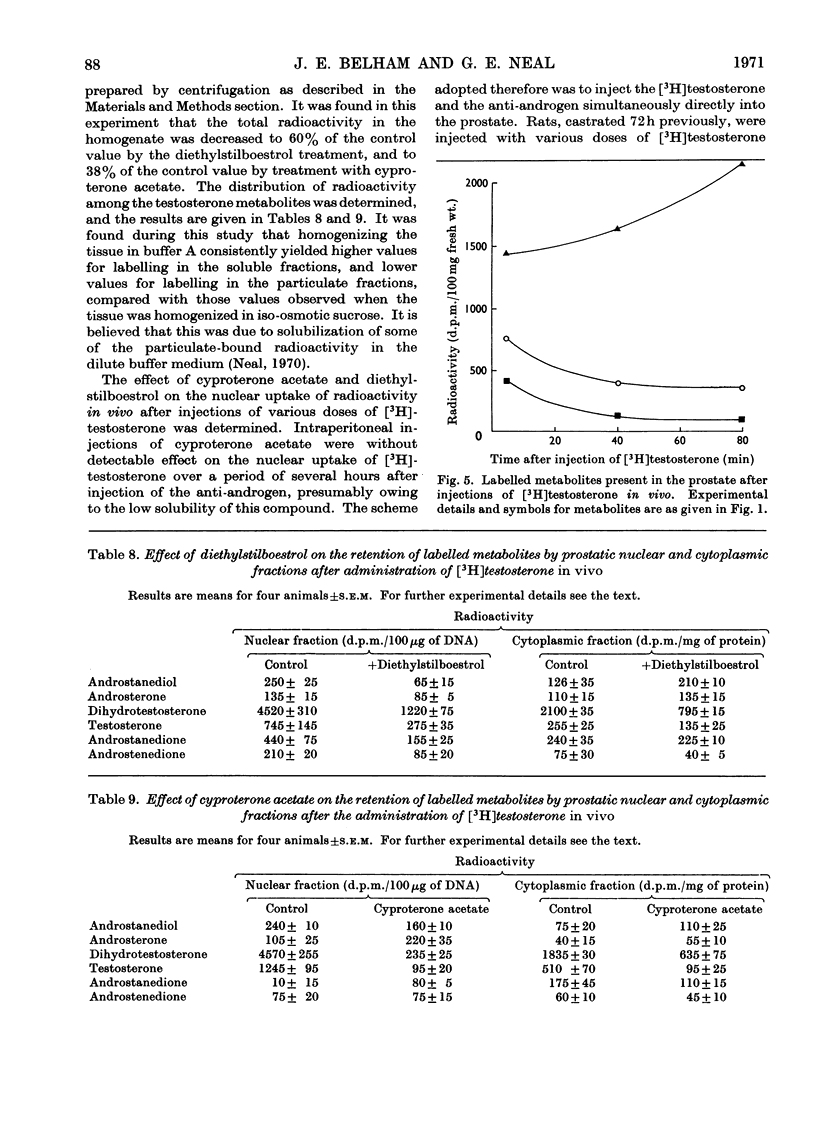
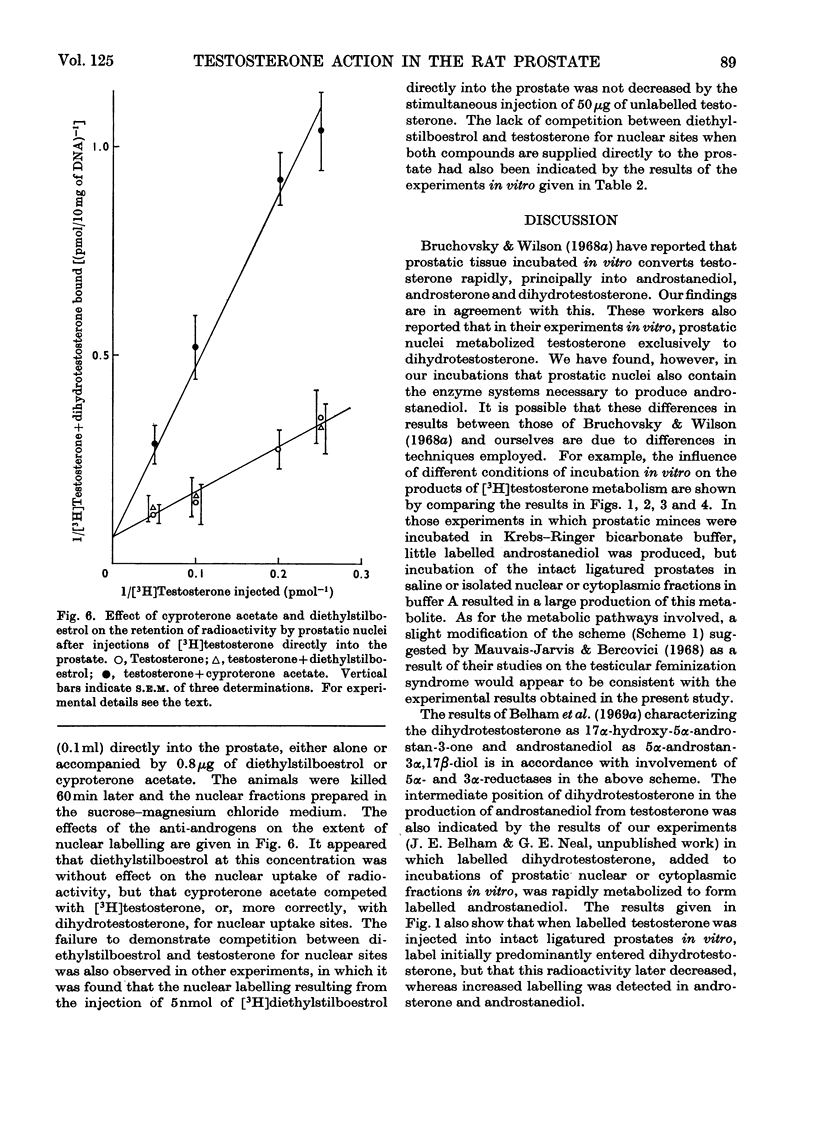
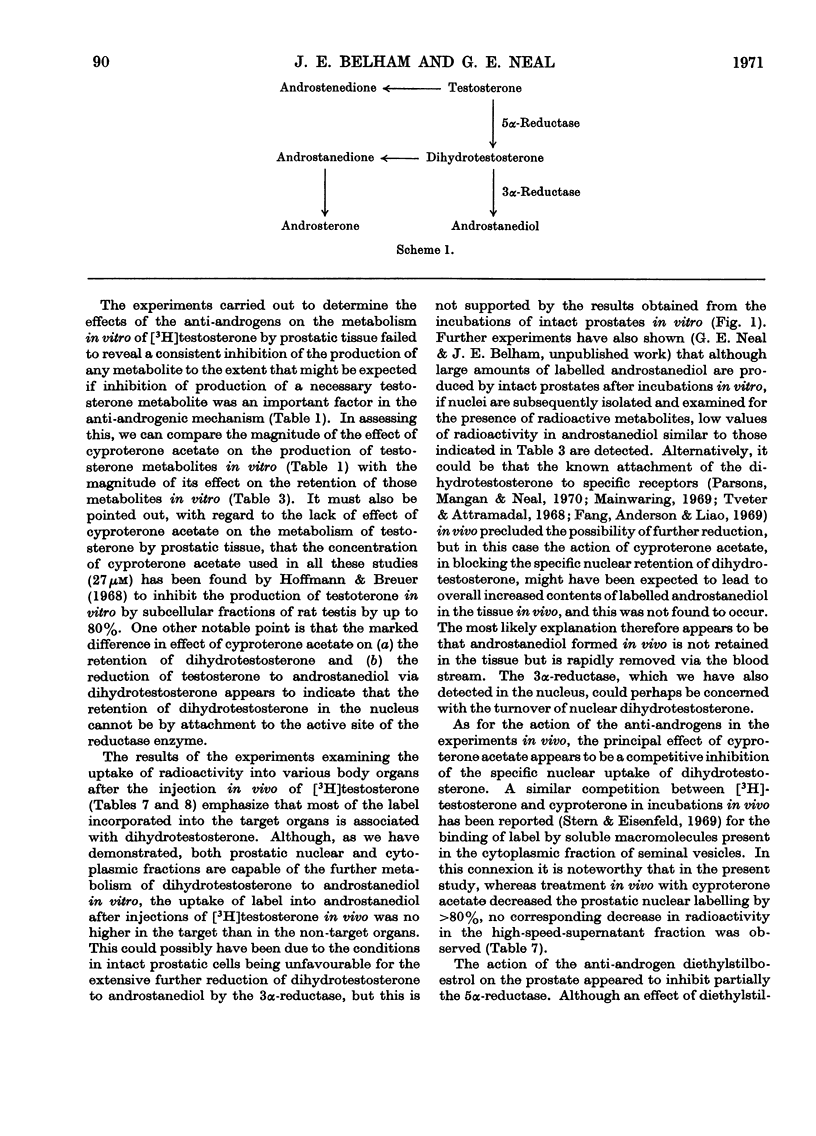
Recent reports have indicated that the prior metabolism of testosterone by the secondary sexual tissues may be necessary for its androgenic effect. The effects of two anti-androgens, diethylstilboestrol and cyproterone acetate (17α-acetoxy-6-chloro-1,2α-methylenepregna-4,6-diene-3,20-dione) used in the chemotherapy of human prostatic carcinoma, have been examined on both the metabolism of testosterone and the retention of its metabolites by the rat ventral prostate gland. Cyproterone acetate was found to inhibit the retention of labelled metabolites of [3H]-testosterone by prostatic nuclei, both in vivo and in vitro. This inhibition appeared to be competitive. In contrast with its effect on nuclear retention of metabolites of testosterone, cyproterone acetate had no significant effect on the metabolism of [3H]testosterone by rat ventral prostate tissue. Diethylstilboestrol similarly had little effect on the metabolism of [3H]testosterone by prostatic tissue, although it did appear partially to inhibit its initial metabolism in all the incubation systems used. Diethylstilboestrol inhibited the nuclear retention of dihydrotestosterone when both [3H]testosterone and diethylstilboestrol were injected intraperitoneally in vivo, but had no effect on dihydrotestosterone retention when both testosterone and diethylstilboestrol were supplied directly to the prostate either in vivo or in vitro. It was concluded that if diethylstilboestrol has an anti-androgenic effect at the level of the target organ as distinct from its effect on androgen production by the testes, then it is probably due to a mechanism differing from that of cyproterone acetate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. M., Liao S. Selective retention of dihydrotestosterone by prostatic nuclei. Nature. 1968 Jul 20;219(5151):277–279. doi: 10.1038/219277a0. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belham J. E., Neal G. E., Williams D. C. Testosterone metabolism in the rat ventral prostate. Biochim Biophys Acta. 1969 Jul 29;187(1):159–162. doi: 10.1016/0005-2760(69)90146-5. [DOI] [PubMed] [Google Scholar]
- Belham J. E., Neal G. E., Williams D. C. The reception of androgens in the rat ventral prostate. Biochem J. 1969 Sep;114(2):32P–32P. doi: 10.1042/bj1140032pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchovsky N., Wilson J. D. The conversion of testosterone to 5-alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J Biol Chem. 1968 Apr 25;243(8):2012–2021. [PubMed] [Google Scholar]
- Bruchovsky N., Wilson J. D. The intranuclear binding of testosterone and 5-alpha-androstan-17-beta-ol-3-one by rat prostate. J Biol Chem. 1968 Nov 25;243(22):5953–5960. [PubMed] [Google Scholar]
- Fang S., Anderson K. M., Liao S. Receptor proteins for androgens. On the role of specific proteins in selective retention of 17-beta-hydroxy-5-alpha-androstan-3-one by rat ventral prostate in vivo and in vitro. J Biol Chem. 1969 Dec 25;244(24):6584–6595. [PubMed] [Google Scholar]
- Fang S., Liao S. Antagonistic action of anti-androgens on the formation of a specific dihydrotestosterone-receptor protein complex in rat ventral prostate. Mol Pharmacol. 1969 Jul;5(4):428–431. [PubMed] [Google Scholar]
- Farnsworth W. E. 10-Demethylation of testosterone by human prostate, in vitro. Steroids. 1965 Nov;6(5):519–530. doi: 10.1016/0039-128x(65)90015-2. [DOI] [PubMed] [Google Scholar]
- Hoffmann W., Breuer H. Wirkung von 1,2lpha-Methylen-6-chlor-delta 4,6-pregnadien-17 alpha-ol-3,20-dion (Cyproteron) auf die Biogenese von C19-Steroiden in Rattentestes. Acta Endocrinol (Copenh) 1968 Apr;57(4):623–638. [PubMed] [Google Scholar]
- KREBS H. A. Body size and tissue respiration. Biochim Biophys Acta. 1950 Jan;4(1-3):249–269. doi: 10.1016/0006-3002(50)90032-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Mainwaring W. I. The binding of (1,2-3H)testosterone within nuclei of the rat prostate. J Endocrinol. 1969 Jul;44(3):323–333. doi: 10.1677/joe.0.0440323. [DOI] [PubMed] [Google Scholar]
- Neal G. E. Androgen uptake by rat ventral prostate. Biochem J. 1970 Jun;118(2):12P–12P. doi: 10.1042/bj1180012pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons I. C., Mangan F. R., Neal G. E. Some effects of testosterone on the rat ventral prostate. Biochem J. 1970 Apr;117(3):425–430. doi: 10.1042/bj1170425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern J. M., Eisenfeld A. J. Androgen accumulation andbinding to macromolecules in seminal vesicles: inhibition cyproterone. Science. 1969 Oct 10;166(3902):233–235. doi: 10.1126/science.166.3902.233. [DOI] [PubMed] [Google Scholar]
- Tveter K. J., Attramadal A. Selective uptake of radioactivity in rat ventral prostate following administration of testosterone-1,2-3H. Methodological considerations. Acta Endocrinol (Copenh) 1968 Oct;59(2):218–226. doi: 10.1530/acta.0.0590218. [DOI] [PubMed] [Google Scholar]
- Widnell C. C., Tata J. R. A procedure for the isolation of enzymically active rat-liver nuclei. Biochem J. 1964 Aug;92(2):313–317. doi: 10.1042/bj0920313. [DOI] [PMC free article] [PubMed] [Google Scholar]


