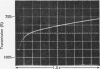
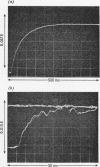
Abstract
1. The hydrolysis of 2,4-dinitrophenyl phosphate by Escherichia coli alkaline phosphatase at pH5.5 was studied by the stopped-flow technique. The rate of production of 2,4-dinitrophenol was measured both in reactions with substrate in excess of enzyme and in single turnovers with excess of enzyme over substrate. It was found that the step that determined the rate of the transient phase of this reaction was an isomerization of the enzyme occurring before substrate binding. 2. No difference was observed between the reaction after mixing a pre-equilibrium mixture of alkaline phosphatase and inorganic phosphate, with 2,4-dinitrophenyl phosphate at pH5.5 in the stopped-flow apparatus, and the control reaction in which inorganic phosphate was pre-equilibrated with the substrate. Since dephosphorylation is the rate-limiting step of the complete turnover at pH5.5, this observation suggests that alkaline phosphatase can bind two different ligands simultaneously, one at each of the active sites on the dimeric enzyme, even though only one site is catalytically active at any given time. 3. Kinetic methods are outlined for the distinction between two pathways of substrate binding, which include an isomerization either of the free enzyme or of the enzyme–substrate complex.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebury M. L., Johnson B. P., Coleman J. E. Phosphate binding to alkaline phosphatase. Metal ion dependence. J Biol Chem. 1970 Oct 10;245(19):4968–4976. [PubMed] [Google Scholar]
- Fernley H. N., Walker P. C. Effect of sodium chloride on Escherichia coli alkaline phosphatase. Biochem J. 1968 Nov;110(2):11P–12P. doi: 10.1042/bj1100011pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernley H. N., Walker P. G. Studies on alkaline phosphatase. Transient-state and steady-state kinetics of Escherichia coli alkaline phosphatase. Biochem J. 1969 Jan;111(2):187–194. doi: 10.1042/bj1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden C. Kinetic aspects of regulation of metabolic processes. The hysteretic enzyme concept. J Biol Chem. 1970 Nov 10;245(21):5788–5799. [PubMed] [Google Scholar]
- GOMORI G. Alkaline phosphatase of cell nuclei. J Lab Clin Med. 1951 Apr;37(4):526–531. [PubMed] [Google Scholar]
- GUTFREUND H., STURTEVANT J. M. The mechanism of the reaction of chymotrypsin with p-nitrophenyl acetate. Biochem J. 1956 Aug;63(4):656–661. doi: 10.1042/bj0630656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutfreund H. Mechanisms of enzyme-catalysed hydrolysis reactions: present status and outstanding problems. Biochem J. 1968 Nov;110(2):2P–3P. doi: 10.1042/bj1100002pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford S. E., Bennett N. G., Trentham D. R., Gutfeund H. A substate-induced conformation change in the reaction of alkaline phosphatase from Escherichia coli. Biochem J. 1969 Sep;114(2):243–251. doi: 10.1042/bj1140243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko S. H., Kézdy F. J. The kinetics of the Escherichia coli alkaline phosphatase catalyzed hydrolysis of 2,4-dinitrophenyl phosphate. J Am Chem Soc. 1967 Dec 20;89(26):7139–7140. doi: 10.1021/ja01002a068. [DOI] [PubMed] [Google Scholar]
- MALAMY M. H., HORECKER B. L. PURIFICATION AND CRYSTALLIZATION OF THE ALKALINE PHOSPHATASE OF ESCHERICHIA COLI. Biochemistry. 1964 Dec;3:1893–1897. doi: 10.1021/bi00900a018. [DOI] [PubMed] [Google Scholar]
- PIGRETTI M. M., MILSTEIN C. ACID INACTIVATION OF AND INCORPORATION OF PHOSPHATE INTO ALKALINE PHOSPHATASE FROM ESCHERICHIA COLI. Biochem J. 1965 Jan;94:106–113. doi: 10.1042/bj0940106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHMAN F., BYRNE R. Fingerprint analysis of alkaline phosphatase of Escherichia coli K12. J Mol Biol. 1963 Apr;6:330–340. doi: 10.1016/s0022-2836(63)80092-3. [DOI] [PubMed] [Google Scholar]
- Reid T. W., Pavlic M., Sullivan D. J., Wilson I. B. The mechanistic significance of phosphate labeling of alkaline phosphatase. Biochemistry. 1969 Aug;8(8):3184–3188. doi: 10.1021/bi00836a008. [DOI] [PubMed] [Google Scholar]
- Reid T. W., Wilson I. B. Conformational isomers of alkaline phosphatase in the mechanism of hydrolysis. Biochemistry. 1971 Feb 2;10(3):380–387. doi: 10.1021/bi00779a004. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Schlesinger M. J. Formation and properties of a tetrameric form of Escherichia coli alkaline phosphatase. Biochemistry. 1969 Nov;8(11):4278–4282. doi: 10.1021/bi00839a008. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Barrett K. The reversible dissociation of the alkaline phosphatase of Escherichia coli. I. Formation and reactivation of subunits. J Biol Chem. 1965 Nov;240(11):4284–4292. [PubMed] [Google Scholar]
- Schlesinger M. J., Olsen R. A new, simple, rapid procedure for purification of Escherichia coli alkaline phosphatase. Anal Biochem. 1970 Jul;36(1):86–90. doi: 10.1016/0003-2697(70)90334-9. [DOI] [PubMed] [Google Scholar]
- Trentham D. R., Gutfreund H. The kinetics of the reaction of nitrophenyl phosphates with alkaline phosphatase from Escherichia coli. Biochem J. 1968 Jan;106(2):455–460. doi: 10.1042/bj1060455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. R., McMurray C. H., Pogson C. I. The active chemical state of D-glyceraldehyde 3-phosphate in its reactions with D-glyceraldehyde 3-phosphate dehydrogenase, aldolase and triose phosphate isomerase. Biochem J. 1969 Aug;114(1):19–24. doi: 10.1042/bj1140019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Williams R. J. Metalloenzymes: the entatic nature of their active sites. Proc Natl Acad Sci U S A. 1968 Feb;59(2):498–505. doi: 10.1073/pnas.59.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]