Abstract
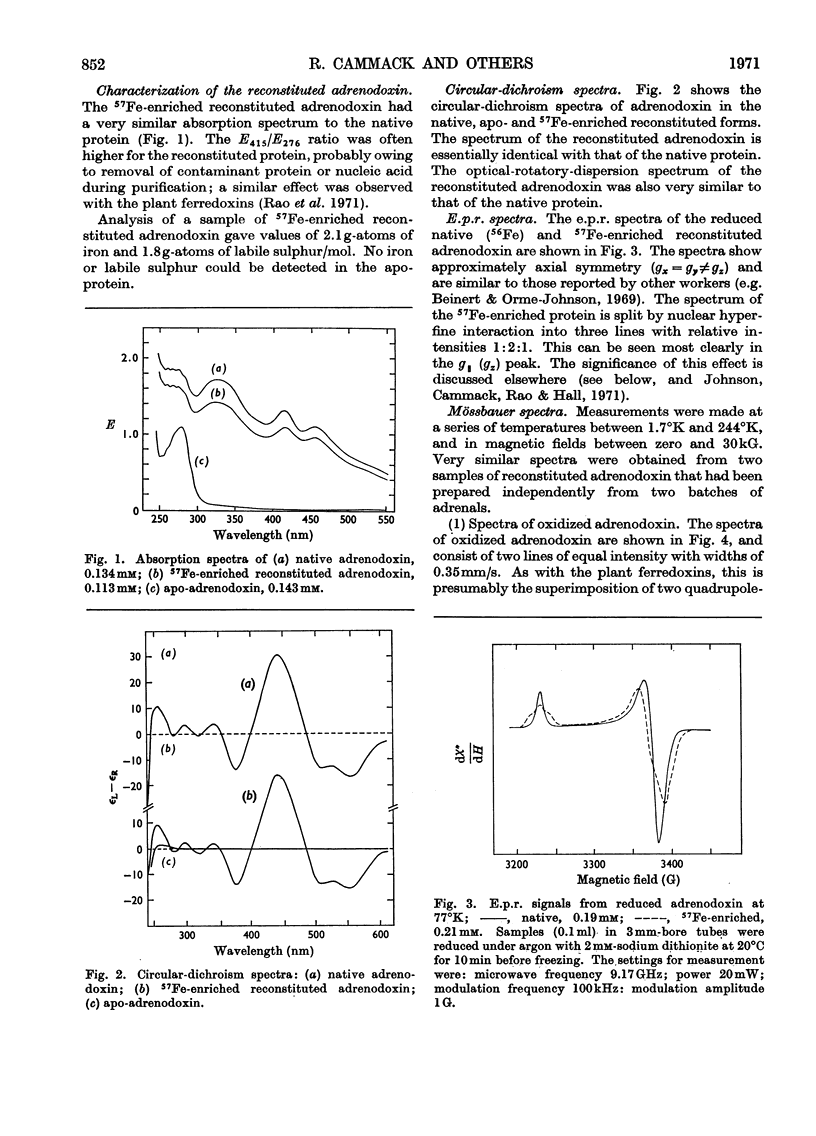
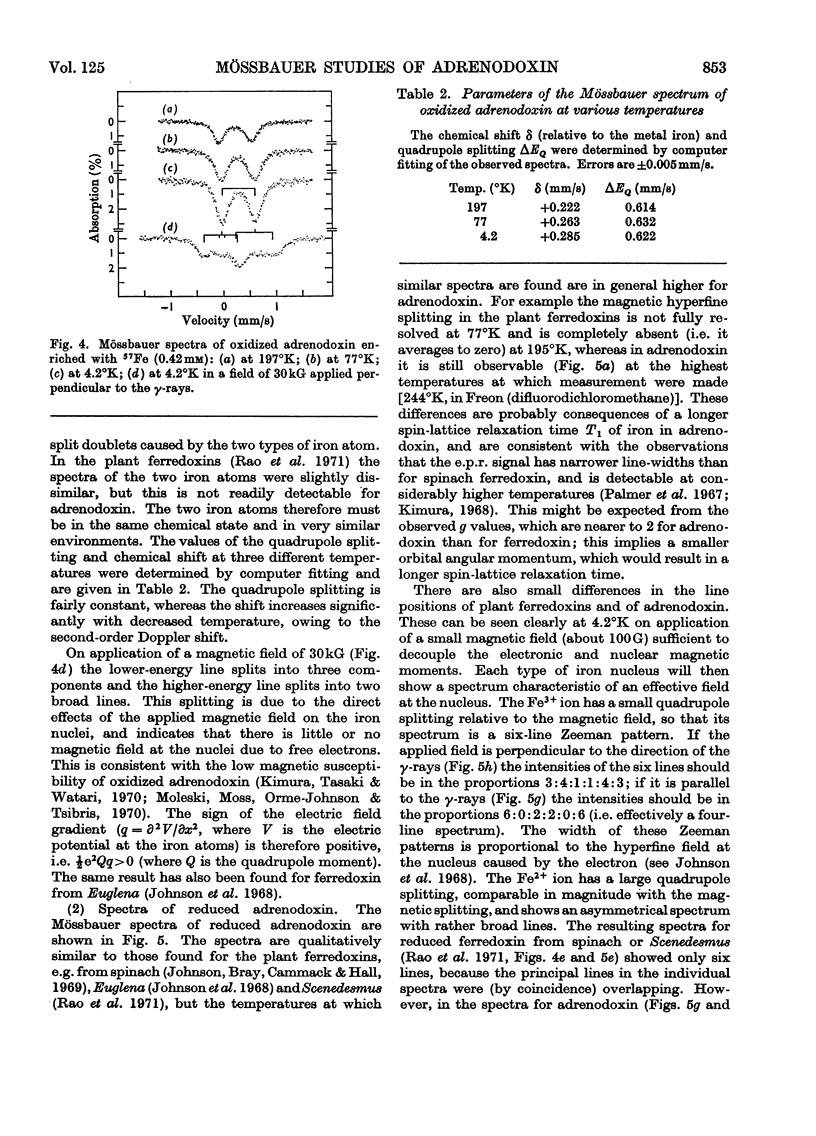
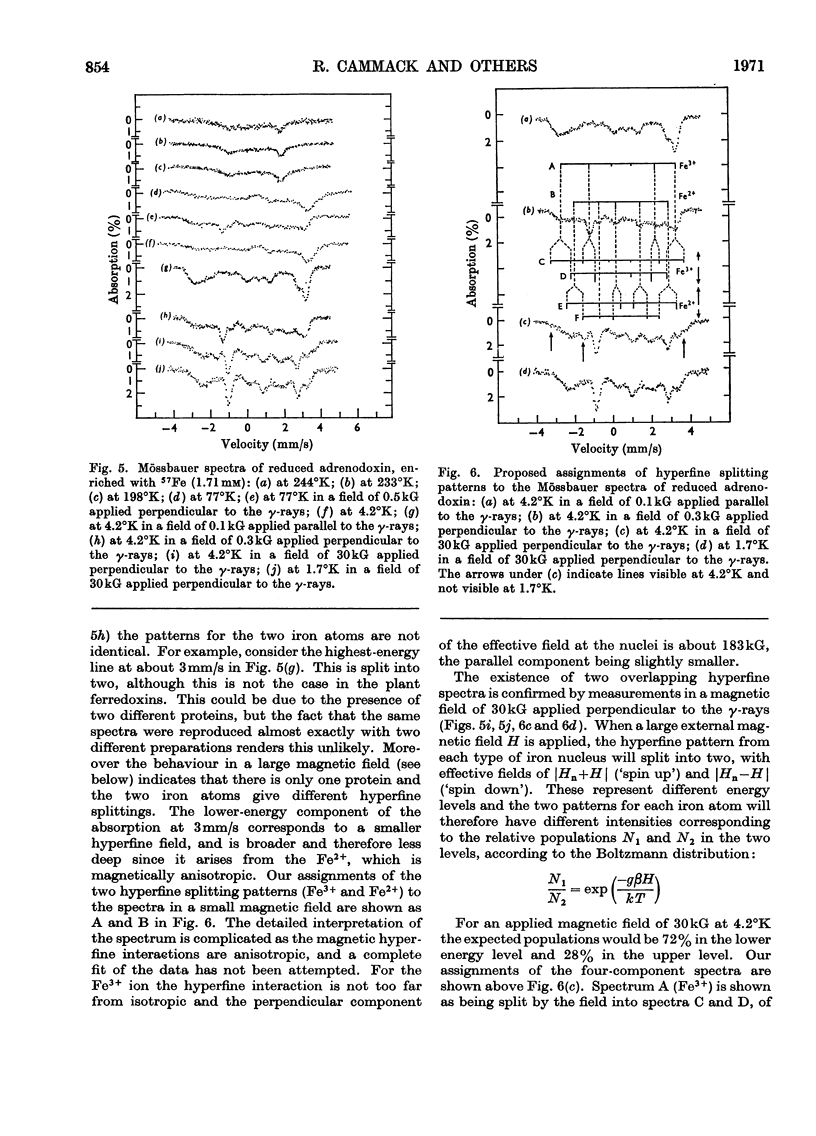
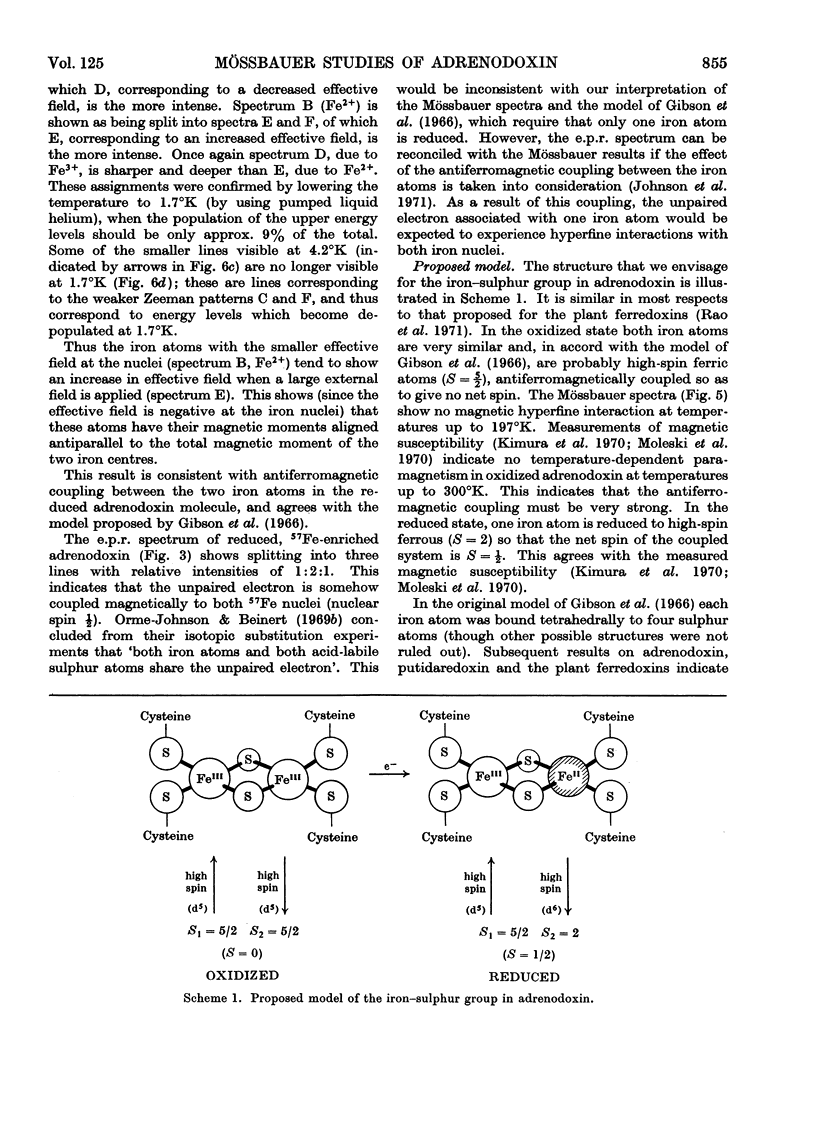
1. Mössbauer spectra were measured of adrenodoxin purified from porcine adrenal glands. They show similarities to the spectra of the plant ferredoxins. All of these proteins contain two atoms of iron and two of inorganic sulphide per molecule, and on reduction accept one electron. 2. As with the plant ferredoxins the adrenodoxin for these measurements was enriched with 57Fe by reconstitution of the apo-protein, and subsequently was carefully purified and checked by a number of methods to ensure that it was in the same conformation as the native protein and contained no extraneous iron. 3. The Mössbauer spectra of oxidized adrenodoxin at temperatures from 4.2°K to 197°K show that the iron atoms are probably high-spin Fe3+, and in similar environments, and experience little or no magnetic field from the electrons. 4. Mössbauer spectra of reduced adrenodoxin showed magnetic hyperfine structure at all temperatures from 1.7°K to 244°K, in contrast with the reduced plant ferredoxins, which showed it only at lower temperatures. This is a consequence of a longer electron-spin relaxation time in reduced adrenodoxin. 5. At 4.2°K in a small magnetic field the spectrum of reduced adrenodoxin shows a sixline Zeeman pattern due to Fe3+ superimposed upon a combined magnetic and quadrupole spectrum due to Fe2+. 6. In a large magnetic field (30kG) each hyperfine pattern is further split into two. Analysis of these spectra at 4.2°K and 1.7°K shows that the effective fields at the Fe3+ and Fe2+ nuclei are in opposite directions. This agrees with the proposal, first made for the ferredoxins, that the iron atoms are antiferromagnetically coupled. 7. In accord with the model for the ferredoxins, it is proposed that the oxidized adrenodoxin contains two high-spin Fe3+ atoms which are antiferromagnetically coupled; on reduction one iron atom becomes high-spin Fe2+.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beinert H., Orme-Johnson W. H. Electron-nuclear and electron-electron spin interactions in the study of enzyme structure and function. Ann N Y Acad Sci. 1969 May 16;158(1):336–360. doi: 10.1111/j.1749-6632.1969.tb56230.x. [DOI] [PubMed] [Google Scholar]
- Cooke R., Tsibris J. C., Debrunner P. G., Tsai R., Gunsalus I. C., Frauenfelder H. Mössbauer studies on putidaredoxin. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1045–1052. doi: 10.1073/pnas.59.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman D. W., Tsai R. L., Gunsalus I. C. The ferroprotein component of a methylene hydroxylase. Biochem Biophys Res Commun. 1967 Mar 9;26(5):577–583. doi: 10.1016/0006-291x(67)90104-0. [DOI] [PubMed] [Google Scholar]
- Eisenstein K. K., Wang J. H. Conversion of light to chemical free energy. I. Porphyrin-sensitized photoreduction of ferredoxin by glutathione. J Biol Chem. 1969 Apr 10;244(7):1720–1728. [PubMed] [Google Scholar]
- Evans M. C., Hall D. O., Johnson C. E. Hyperfine structure of (57Fe) iron in the Mössbauer spectrum of the high-potential iron protein from Chromatium. Biochem J. 1970 Sep;119(2):289–291. doi: 10.1042/bj1190289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. F., Hall D. O., Thornley J. H., Whatley F. R. The iron complex in spinach ferredoxin. Proc Natl Acad Sci U S A. 1966 Sep;56(3):987–990. doi: 10.1073/pnas.56.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. O., Evans M. C. Iron-sulphur proteins. Nature. 1969 Sep 27;223(5213):1342–1348. doi: 10.1038/2231342a0. [DOI] [PubMed] [Google Scholar]
- Johnson C. E., Bray R. C., Cammack R., Hall D. O. Mossbauer spectroscopy of the iron-sulfur proteins. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1234–1238. doi: 10.1073/pnas.63.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. E., Cammack R., Rao K. K., Hall D. O. The interpretation of the EPR and Mössbauer spectra of two-iron, one-electron, iron-sulphur proteins. Biochem Biophys Res Commun. 1971 May 7;43(3):564–571. doi: 10.1016/0006-291x(71)90651-6. [DOI] [PubMed] [Google Scholar]
- Johnson C. E., Elstner E., Gibson J. F., Benfield G., Evans M. C., Hall D. O. Mössbauer effect in the ferredoxin of Euglena. Nature. 1968 Dec 28;220(5174):1291–1293. doi: 10.1038/2201291a0. [DOI] [PubMed] [Google Scholar]
- Kimura T., Tasaki A., Watari H. Studies on adrenal steroid hydroxylases. The magnetic susceptibility of oxidized and reduced adrenal iron-sulfur protein (adrenodoxin). J Biol Chem. 1970 Sep 10;245(17):4450–4452. [PubMed] [Google Scholar]
- LOVENBERG W., BUCHANAN B. B., RABINOWITZ J. C. STUDIES ON THE CHEMICAL NATURE OF CLOSTRIDIAL FERREDOXIN. J Biol Chem. 1963 Dec;238:3899–3913. [PubMed] [Google Scholar]
- Moleski C., Moss T. H., Orme-Johnson W. H., Tsibris J. C. The magnetic susceptibility of the oxidized and reduced iron-sulfur proteins adrenodoxin and putidaredoxin. Biochim Biophys Acta. 1970 Sep 29;214(3):548–550. doi: 10.1016/0005-2795(70)90315-6. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson W. H., Beinert H. Heterogeneity of paramagnetic species in two iron-sulfur proteins: Clostridium pasteurianum ferredoxin and milk xanthine oxidase. Biochem Biophys Res Commun. 1969 Aug 7;36(3):337–344. doi: 10.1016/0006-291x(69)90569-5. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson W. H., Beinert H. Reductive titrations of iron-sulfur proteins containing two to four iron atoms. J Biol Chem. 1969 Nov 25;244(22):6143–6148. [PubMed] [Google Scholar]
- Orme-Johnson W. H., Hansen R. E., Beinert H., Tsibris J. C., Bartholomaus R. C., Gunsalus I. C. On the sulfur components of iron-sulfur proteins. I. The number of acid-labile sulfur groups sharing an unpaired electron with iron. Proc Natl Acad Sci U S A. 1968 Jun;60(2):368–372. doi: 10.1073/pnas.60.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G., Brintzinger H., Estabrook R. W. Spectroscopic studies on spinach ferredoxin and adrenodoxin. Biochemistry. 1967 Jun;6(6):1658–1664. doi: 10.1021/bi00858a012. [DOI] [PubMed] [Google Scholar]
- Poe M., Phillips W. D., McDonald C. C., Lovenberg W. Proton magnetic resonance study of ferredoxin from Clostridium pasteurianum. Proc Natl Acad Sci U S A. 1970 Apr;65(4):797–804. doi: 10.1073/pnas.65.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K. K., Cammack R., Hall D. O., Johnson C. E. Mössbauer effect in Scenedesmus and spinach ferredoxins. The mechanism of electron transfer in plant-type iron-sulphur proteins. Biochem J. 1971 Apr;122(3):257–265. doi: 10.1042/bj1220257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Haniu M., Yasunobu K. T. The primary structure of bovine adrenodoxin. Biochem Biophys Res Commun. 1970;39(6):1182–1188. doi: 10.1016/0006-291x(70)90685-6. [DOI] [PubMed] [Google Scholar]
- Thornley J. H., Gibson J. F., Whatley F. R., Hall D. O. Comment on a recent model of the iron complex in spinach ferredoxin. Biochem Biophys Res Commun. 1966 Sep 22;24(6):877–879. doi: 10.1016/0006-291x(66)90330-5. [DOI] [PubMed] [Google Scholar]


