Abstract
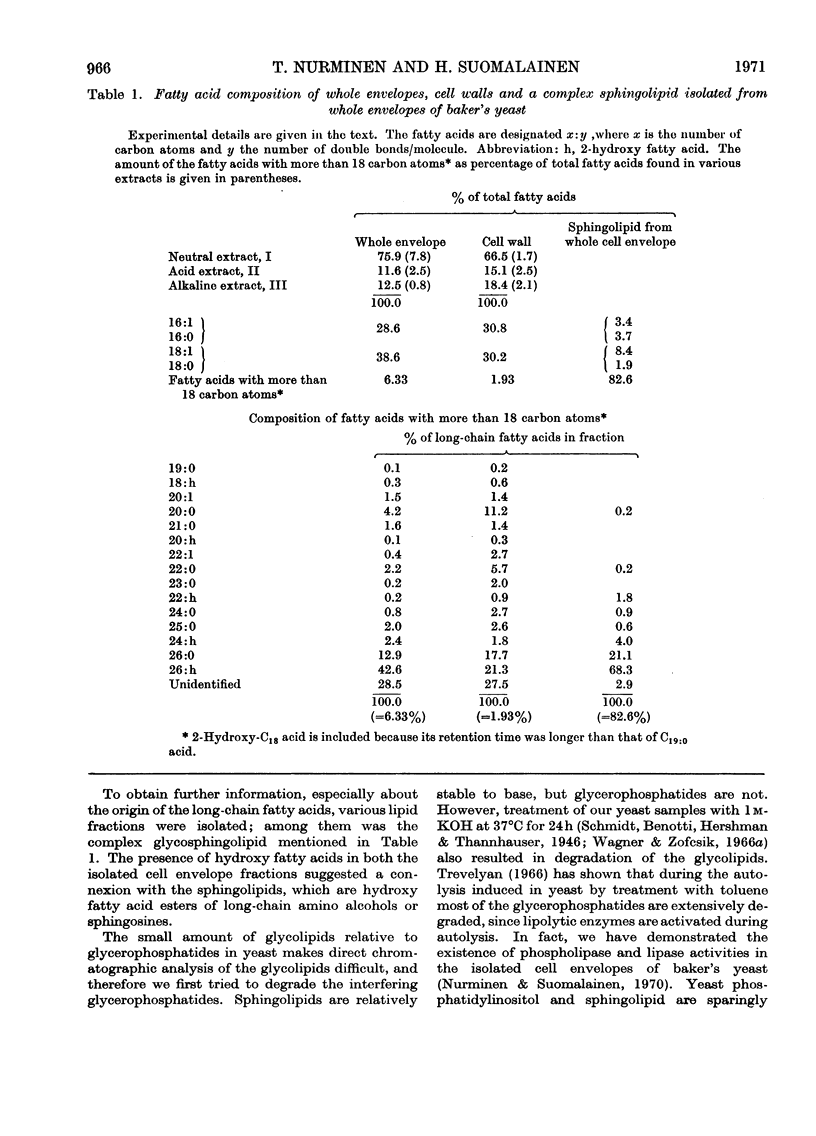
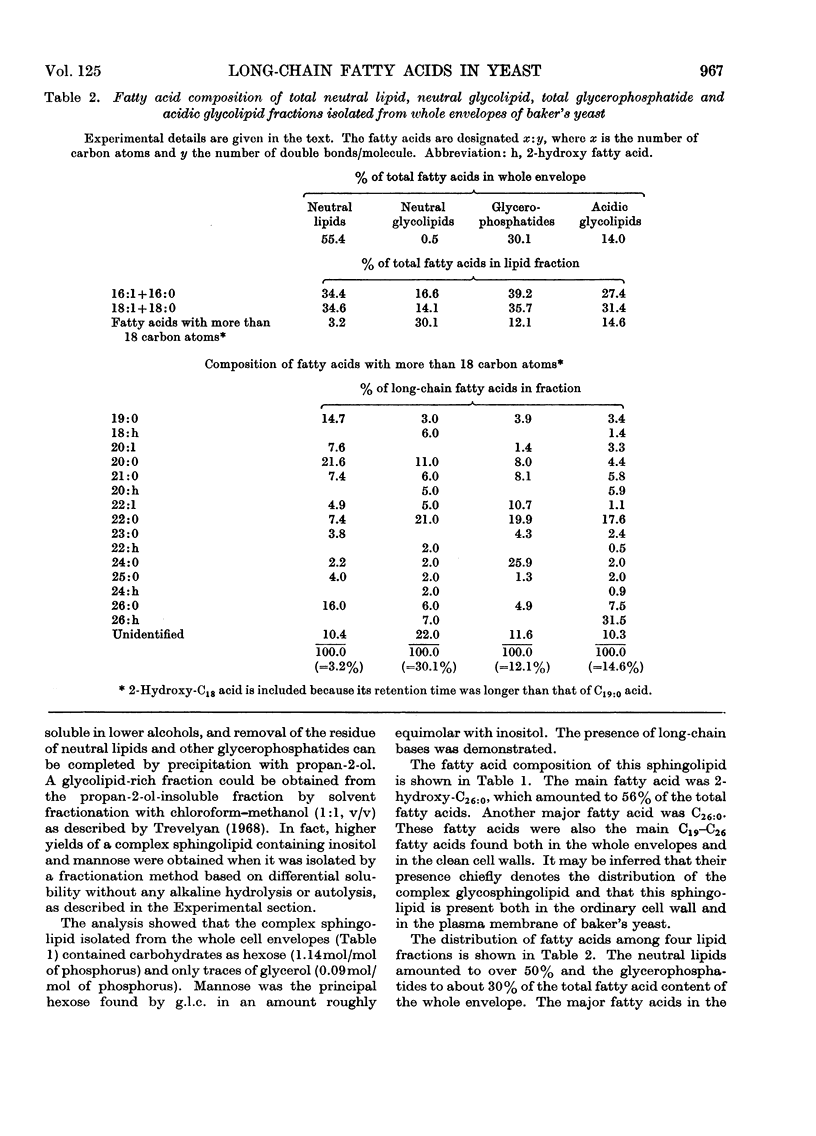
1. The total yield of fatty acids from the whole envelopes was markedly higher than that obtained from the ordinary cell walls. In both samples the major fatty acids were C16 and C18 acids. 2. The whole envelopes contained C18 acids and long-chain (C19–C26) fatty acids, in a higher proportion than did the ordinary cell walls. Fifteen fatty acids with more than 18 carbon atoms were identified, among which 2-hydroxy-C26:0 and C26:0 acids predominated. 3. A complex sphingolipid containing inositol, phosphorus and mannose was isolated from the whole cell envelopes. The main fatty acids of this lipid were 2-hydroxy-C26:0 and C26:0 acids. It was concluded that this sphingolipid is present both in the ordinary cell wall and in the plasma membrane of baker's yeast. 4. The neutral lipids amounted to over 50% and the glycerophosphatides to about 30% of the total fatty acid content of the whole envelope. The major fatty acids in these lipids were C16:1, C18:1 and C16:0 acids. The proportion of fatty acids with more than 18 carbon atoms was lowest in the neutral lipids, whereas the neutral glycolipids contained the highest percentage of these fatty acids. Acidic glycolipids amounted to 14% of the total fatty acid content of the whole envelope. The presence of a cerebroside sulphate in this lipid fraction was demonstrated, whereas the high content of 2-hydroxy-C26:0 acid found is caused by the complex inositol- and mannose-containing sphingolipid.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baraud J., Maurice A., Napias C. Composition et répartition des lipides au sein des cellules de Saccharomyces cerevisiae. Bull Soc Chim Biol (Paris) 1970;52(4):421–432. [PubMed] [Google Scholar]
- Brennan P. J., Flynn M. P., Griffin P. F.S. Acylglucoses in Escherichia coli, Saccharomyces cerevisiae and Agaricus bisporus. FEBS Lett. 1970 Jul 3;8(6):322–324. doi: 10.1016/0014-5793(90)80004-3. [DOI] [PubMed] [Google Scholar]
- Carter H. E., Gaver R. C. Improved reagent for trimethylsilylation of sphingolipid bases. J Lipid Res. 1967 Jul;8(4):391–395. [PubMed] [Google Scholar]
- Carter H. E., Weber E. J. Preparation and properties of various salt forms of plant phosphatidyl inositols. Lipids. 1966 Jan;1(1):16–20. doi: 10.1007/BF02668119. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gonzalez-Sastre F., Folch-Pi J. Thin-layer chromatography of the phosphoinositides. J Lipid Res. 1968 Jul;9(4):532–533. [PubMed] [Google Scholar]
- KATES M. SIMPLIFIED PROCEDURES FOR HYDROLYSIS OR METHANOLYSIS OF LIPIDS. J Lipid Res. 1964 Jan;5:132–135. [PubMed] [Google Scholar]
- KOLB J. J., WEIDNER M. A., TOENNIES G. Microdetermination of lipid phosphorus as a measure of bacterial membrane substance. Anal Biochem. 1963 Jan;5:78–82. doi: 10.1016/0003-2697(63)90061-7. [DOI] [PubMed] [Google Scholar]
- Kayser S. G., Patton S. The function of very long chain fatty acids in membrane structure: evidence from milk cerebrosides. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1572–1578. doi: 10.1016/0006-291x(70)90567-x. [DOI] [PubMed] [Google Scholar]
- Kean E. L. Rapid, sensitive spectrophotometric method for quantitative determination of sulfatides. J Lipid Res. 1968 May;9(3):319–327. [PubMed] [Google Scholar]
- Lester R. L., Steiner M. R. The occurrence of diphosphoinositide and triphosphoinositide in Saccharomyces cerevisiae. J Biol Chem. 1968 Sep 25;243(18):4889–4893. [PubMed] [Google Scholar]
- Nurminen T., Oura E., Suomalainen H. The enzymic composition of the isolated cell wall and plasma membrane of baker's yeast. Biochem J. 1970 Jan;116(1):61–69. doi: 10.1042/bj1160061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminen T., Suomalainen H. The lipolytic activities of the isolated cell envelope fracttions of Baker's yeast. Biochem J. 1970 Aug;118(5):759–763. doi: 10.1042/bj1180759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penick R. J., McCluer R. H. Quantitative determination of glucose and galactose in gangliosides by gas-liquid chromatography. Biochim Biophys Acta. 1966 Apr 4;116(2):288–295. doi: 10.1016/0005-2760(66)90011-7. [DOI] [PubMed] [Google Scholar]
- Shaw N. Bacterial glycolipids. Bacteriol Rev. 1970 Dec;34(4):365–377. doi: 10.1128/br.34.4.365-377.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevelyan W. E. Preparation of phosphatidyl inositol from baker's yeast. J Lipid Res. 1966 May;7(3):445–447. [PubMed] [Google Scholar]


