Abstract
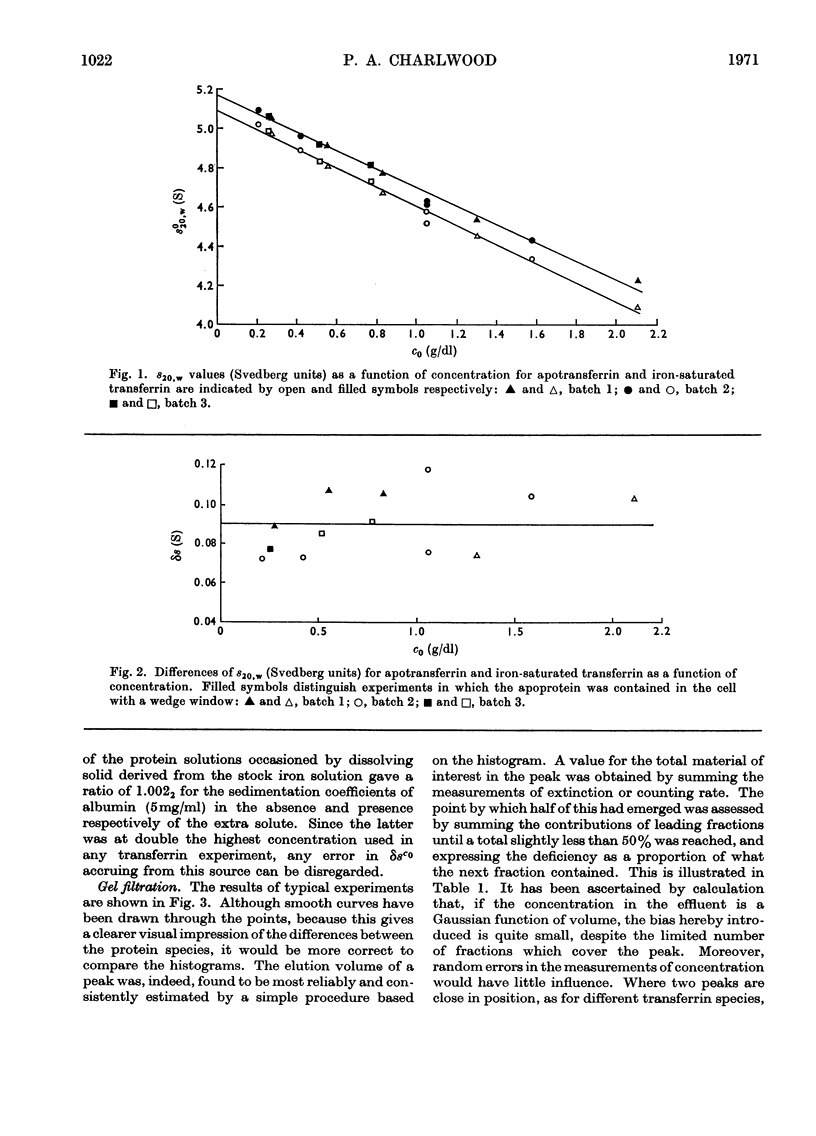
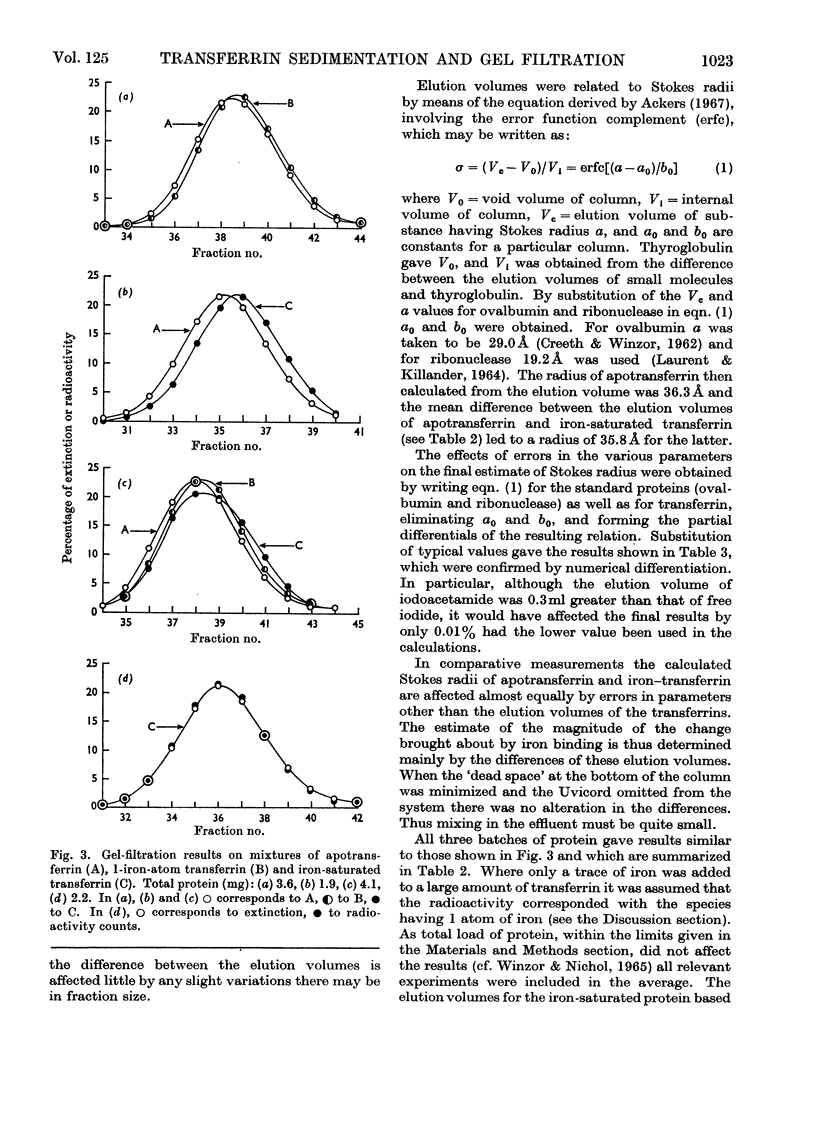
Differential measurements of sedimentation velocity showed that binding of 2 atoms of iron per molecule of human apotransferrin caused an increase in s020,w of about 1.8%. Gel-filtration experiments to compare the elution volumes of apotransferrin and transferrin radioactively labelled with iron showed that binding of the first atom to a molecule produced a decrease in Stokes radius of about 0.7%, and the binding of a second atom an equal decrement. These results confirmed that saturation of human transferrin with iron alters the conformation sufficiently to produce detectable changes in the hydrodynamic properties. They also indicate that the local changes brought about by successive addition of 2 atoms of iron are very similar, if not identical.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P. Citrate-mediated exchange of FE3+ among tranferrin molecules. Biochem Biophys Res Commun. 1968 Jul 26;32(2):220–226. doi: 10.1016/0006-291x(68)90372-0. [DOI] [PubMed] [Google Scholar]
- Aisen P., Koenig S. H., Schillinger W. E., Scheinberg I. H., Mann K. G., Fish W. Absence of dimers and nature of iron binding in transferrin solutions. Nature. 1970 May 30;226(5248):859–861. doi: 10.1038/226859a0. [DOI] [PubMed] [Google Scholar]
- Aisen P., Leibman A., Reich H. A. Studies on the binding of iron to transferrin and conalbumin. J Biol Chem. 1966 Apr 25;241(8):1666–1671. [PubMed] [Google Scholar]
- Bates G. W., Billups C., Saltman P. The kinetics and mechanism of iron (3) exchange between chelates and transferrin. I. The complexes of citrate and nitrilotriacetic acid. J Biol Chem. 1967 Jun 25;242(12):2810–2815. [PubMed] [Google Scholar]
- Bates G. W., Billups C., Saltman P. The kinetics and mechanism of iron (3) exchange between chelates and transferrin. II. The presentation and removal with ethylenediaminetetraacetate. J Biol Chem. 1967 Jun 25;242(12):2816–2821. [PubMed] [Google Scholar]
- Bezkorovainy A. Comparative study of metal-free, iron-saturated and sialic acid-free transferrins. Biochim Biophys Acta. 1966 Oct 31;127(2):535–537. doi: 10.1016/0304-4165(66)90410-7. [DOI] [PubMed] [Google Scholar]
- Bezkorovainy A. Ultracentrifugal behavior of transferrin in the presence of some anions. Biochim Biophys Acta. 1966 Oct 10;126(2):286–291. doi: 10.1016/0926-6585(66)90065-3. [DOI] [PubMed] [Google Scholar]
- Bloomfield V. The structure of bovine serum albumin at low pH. Biochemistry. 1966 Feb;5(2):684–689. doi: 10.1021/bi00866a039. [DOI] [PubMed] [Google Scholar]
- Brownstone A. D. A versatile system for preparative electrophoresis in acrylamide gel. Anal Biochem. 1969 Jan;27(1):25–46. doi: 10.1016/0003-2697(69)90216-4. [DOI] [PubMed] [Google Scholar]
- CHARLWOOD P. A. Applications of radioactively labeled marker proteins in density gradient ultracentrifugation. Anal Biochem. 1963 Mar;5:226–245. doi: 10.1016/0003-2697(63)90120-9. [DOI] [PubMed] [Google Scholar]
- CHARLWOOD P. A. ULTRACENTRIFUGAL CHARACTERISTICS OF HUMAN, MONEKY AND RAT TRANSFERRINS. Biochem J. 1963 Sep;88:394–398. doi: 10.1042/bj0880394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlwood P. A. Isolation of human beta1A-globulin by modern techniques. Biochem J. 1969 Dec;115(5):897–902. doi: 10.1042/bj1150897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J., Huehns E. R. Significance of the binding of iron by transferrin. Nature. 1967 Aug 5;215(5101):584–586. doi: 10.1038/215584a0. [DOI] [PubMed] [Google Scholar]
- GORDON A. H., LOUIS L. N. PREPARATION AND PROPERTIES OF RAT TRANSFERRIN. Biochem J. 1963 Sep;88:409–414. doi: 10.1042/bj0880409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene F. C., Feeney R. E. Physical evidence for transferrins as single polypeptide chains. Biochemistry. 1968 Apr;7(4):1366–1371. doi: 10.1021/bi00844a018. [DOI] [PubMed] [Google Scholar]
- KATZ J. H., ZOUKIS M., HART W. L., DERN R. J. A SIMPLIFIED PROCEDURE FOR THE SIMULTANEOUS ASSAY OF FE55 AND FE59 IN A LIQUID SCINTILLATION SYSTEM. J Lab Clin Med. 1964 May;63:885–893. [PubMed] [Google Scholar]
- Kirschner M. W., Schachman H. K. Conformational changes in proteins as measured by difference sedimentation studies. I. A technique for measuring small changes in sedimentation coefficient. Biochemistry. 1971 May 11;10(10):1900–1919. doi: 10.1021/bi00786a027. [DOI] [PubMed] [Google Scholar]
- Lane R. S. DEAE-cellulose chromatography of human transferrin: the effect of increasing iron saturation and copper(II) binding. Biochim Biophys Acta. 1971 Aug 27;243(2):193–202. doi: 10.1016/0005-2795(71)90076-6. [DOI] [PubMed] [Google Scholar]
- Mann K. G., Fish W. W., Cox A. C., Tanford C. Single-chain nature of human serum transferrin. Biochemistry. 1970 Mar 17;9(6):1348–1354. doi: 10.1021/bi00808a008. [DOI] [PubMed] [Google Scholar]
- PARKER W. C., BEARN A. G. Studies on the transferrins of adult serum, cord serum, and cerebrospinal fluid. The effect of neuraminidase. J Exp Med. 1962 Jan 1;115:83–105. doi: 10.1084/jem.115.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R., Makey D. G., Seal U. S. Human transferrin. Molecular weight and sedimentation properties. J Biol Chem. 1966 Nov 10;241(21):4907–4913. [PubMed] [Google Scholar]
- Rodbard D., Chrambach A. Unified theory for gel electrophoresis and gel filtration. Proc Natl Acad Sci U S A. 1970 Apr;65(4):970–977. doi: 10.1073/pnas.65.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosseneu-Motreff M. Y., Soetewey F., Lamote R., Peeters H. Size and shape determination of apotransferrin and transferrin monomers. Biopolymers. 1971 Jun;10(6):1039–1048. doi: 10.1002/bip.360100610. [DOI] [PubMed] [Google Scholar]
- Schumaker V., Adams P. Differential sedimentation coefficients. I. Precise measurement. Determination of concentration dependence for IgG-immunoglobulin. Biochemistry. 1968 Oct;7(10):3422–3427. doi: 10.1021/bi00850a017. [DOI] [PubMed] [Google Scholar]
- WINZOR D. J., CREETH J. M. Physicochemical studies on ovalbumin. 3. The sulphydryl and disulphide contents of ovalbumin and an iodine-modified derivative. Biochem J. 1962 Jun;83:559–566. doi: 10.1042/bj0830559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenn R. V., Williams J. The isoelectric fractionation of hen's-egg ovotransferrin. Biochem J. 1968 Jun;108(1):69–74. doi: 10.1042/bj1080069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzor D. J., Nichol L. W. Effects of concentration-dependence in gel filtration. Biochim Biophys Acta. 1965 Jun 15;104(1):1–10. doi: 10.1016/0304-4165(65)90213-8. [DOI] [PubMed] [Google Scholar]


