Abstract
Background
Poly (ADP-ribose) polymerase inhibitors (PARPi) serve as crucial therapeutic agents in solid tumor treatment. Preclinical investigations suggest a potential protective function of PARPi against endocrine and metabolic impairments. Nevertheless, the existing body of evidence remains inconclusive on this aspect.
Purpose
Our aim was to evaluate the potential impact of PARPi on endocrine and metabolic disruptions in clinical trials.
Data sources
We conducted a comprehensive search across the Medline, EMBASE, PubMed, and Web of Science databases, along with the ClinicalTrials.gov registry.
Study selection
Phase II/III randomized controlled trials (RCTs) investigating the effects of PARPi in metabolic and endocrine processes were selected for inclusion in patients with solid tumors.
Data extraction
The primary outcomes of interest encompassed metabolic and endocrine dysfunctions.
Data synthesis
A total of 26 trials (n = 9,590 patients) were included in our meta-analysis. Niraparib demonstrated an increased risk of any-grade hyperglycemia (OR = 2.15, 95% CI 1.28–3.62), with patients receiving PARPi for metastatic pancreatic cancer showing a higher susceptibility to any-grade hyperglycemia (OR = 1.78, 95% CI 1.04–3.04). Conversely, rucaparib exhibited a potential ameliorative effect on hyperglycemia (OR = 0.54, 95% CI 0.30–0.97). No statistically significant disparities were observed for other outcomes associated with PARPi utilization.
Limitations
Among these RCTs included, 50% were assessed as low qualities due to high risk of bias.
Conclusions
Our meta-analysis demonstrated that PARPi may exert adverse effects on endocrine and metabolic pathways. Close monitoring of hyperglycemia is recommended for patients undergoing niraparib therapy, especially those with pancreatic cancer.
Trial registration
This meta-Analysis was prospectively registered in the PROSPERO database with ID CRD42023457959.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-025-13579-1.
Keywords: PARP inhibitors, Endocrine and metabolic abnormalities, Meta-analysis
Highlights
Preclinical studies indicate that PARPi may provide protection against endocrine and metabolic abnormalities.
This systematic review and meta-analysis aimed to assess the association between PARPi and the incidence of endocrine and metabolic abnormalities in patients with solid tumors.
Our findings revealed that PARPi adversely affect both endocrine and metabolic processes in patients with solid tumors.
Special attention is warranted regarding hyperglycemia in patients treated with niraparib, particularly those with pancreatic cancer.
The underlying mechanisms of PARPi’s effects on endocrine and metabolic functions remain unclear, highlighting the need for further comprehensive research.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-025-13579-1.
Introduction
Poly (ADP-ribose) polymerase inhibitors (PARPi) have significantly transformed the landscape of oncology therapeutics, extending their application beyond the targeted treatment of DNA repair deficiencies to a diverse array of solid tumors [1, 2]. Initially recognized for their synthetic lethality in BRCA1/2-related cancers, these inhibitors have demonstrated expanded clinical efficacy across various cancer types. Their established role in ovarian cancer, breast cancer, lung cancers, and metastatic castration-resistant prostate cancer (mCRPC) is noteworthy [3–5].
Recent studies highlight the dynamic evolution of systemic treatment with PARPi, emphasising their potential in pancreatic cancer (PC) and gastric cancer (GC) [6, 7]. These studies, combined with the growing body of evidence on the safety and efficacy of PARPi in mCRPC [6, 8], facilitate a deeper understanding of their integration into clinical practice. Despite their therapeutic advantages, PARPi are associated with significant toxicities, as highlighted in various studies, necessitating meticulous consideration and management in clinical environments [2, 6]. However, the precise effects of PARPi on human endocrine and metabolic systems remain unknown, highlighting a significant research gap that should be addressed in clinical oncology studies.
PARPi induce synthetic lethality in cancer cells by inhibiting the catalytic activity of PARP enzymes. These therapeutic agents primarily target PARP1, a pivotal enzyme, as well as PARP2, PARP3, and PARP13 [1]. In preclinical investigations, PARP1 regulates essential cellular functions encompassing inflammation, transcriptional regulation, and cellular bioenergetics, all intertwined with the pathogenesis of endocrine and metabolic disorders [9].
In a clinical trial evaluating olaparib for ovarian cancer, a subset of patients encountered hypothyroidism [10, 11]. Additionally, instances of hyperglycemia have surfaced among patients receiving PARPi, including olaparib [12] and rucaparib [13]. Emerging evidence suggests that PARPi, such as olaparib and rucaparib, might impact lipid metabolism, potentially precipitating dyslipidemia [13, 14]. While endocrine and metabolic complications with PARPi are uncommon and not universal among patients, the scarcity of robust clinical evidence persists [15].
The current meta-analysis comprehensively assessed the metabolic and endocrine effects of PARPi therapy in patients with solid tumors, synthesizing evidence from key Phase II and III randomised controlled trials. This study significantly enriches the oncological conversation by elucidating the intricate interplay between PARPi treatment and the endocrine and metabolic profiles of cancer patients, offering valuable insights for enhancing clinical strategies and optimizing patient care.
Methods
Data sources and searches
The protocol for this meta-analysis was registered in the International Prospective Register of Systematic Reviews online database (CRD42023457959, https://www.crd.york.ac.uk/PROSPERO/#recordDetails). Adhering to PRISMA’s (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) reporting guidelines [16], we conducted a comprehensive search across various electronic databases, including Medline, EMBASE, PubMed, and Web of Science, as well as the ClinicalTrials.gov registry. The search spanned from January 1, 1988, to November 18, 2023, with a language restriction to English.
To identify pertinent studies in humans, we employed search terms ‘PARP’, ‘olaparib’, ‘rucaparib’, ‘niraparib’, ‘talazoparib’, ‘veliparib’, ‘iniparib’, ‘fuzuloparib’, and ‘randomized controlled trials’ (supplementary material Table 1). The search process was independently performed by two authors (via SL F and JY C). In addition, grey literature and conference abstracts meeting inclusion criteria were searched for full-text articles. Safety data sourced from ClinicalTrials.gov, lacking a corresponding publication, were also considered for inclusion. The relevant literature was meticulously managed using the document management software, EndNote version X9.
Study selection
The selection of references for data extraction eligibility was independently carried out by two writers, ZY F. and PJ Z. In cases of disagreement, a third author, MH C, was consulted to facilitate consensus. The study’s inclusion criteria consisted of randomized controlled trials that compared the use of PARPi with either placebo or non-placebo controls, guided by the PICO framework.
The population (P) consisted of adult patients aged 18 and above diagnosed with solid cancer. The intervention (I) involved treatments with PARPi. A comparison (C) was made between therapy with and without PARPi, distinguishing between placebo and non-placebo interventions. The outcomes (O) encompassed metabolic and endocrine dysfunctions, including hyper- or hypoglycemia, type 2 diabetes, hyper- or hypothyroidism, hypertriglyceridemia, and hypercholesterolemia.
Excluded from consideration were case studies, observational studies, non-randomised trials, reviews and guidelines, investigations, conference articles, Phase I studies, and trials with PARPi in both groups. In the absence of adverse events reports on ClinicalTrials.gov, occurrences of endocrine and metabolic dysfunctions were extracted from published RCTs. If such data were unavailable on ClinicalTrials.gov or in publications, efforts were made to obtain them by contacting the study authors or sponsors. RCTs lacking sufficient information on relevant adverse events were omitted from the analysis.
Data extraction
The inclusion of studies for data extraction was meticulously conducted by three authors (L Q, CC G, and ZY F), who extensively reviewed full texts, abstracts, and titles. Data abstraction was performed by four authors (SL F, PJ Z, XX Z, and ZY F) in pairs, with any discrepancies resolved through consultation with B L, MH C, and JY L. Covidence, an online software by Veritas Health Innovation, facilitated the data abstraction process. Key data points collected for each study included the study name, publication year, trial phase, number of enrolled patients, type of solid tumor, and the therapies given to both the intervention and control groups. Authors were contacted at least twice to address any missing or unclear information, ensuring data accuracy and completeness.
Risk of bias assessment
The quality assessment of RCTs was independently conducted by two investigators (SL F and PJ Z) using the Cochrane Risk of Bias 2.0 Tool, integrated with RevMan_V5.4 21 [17]. This tool assessed RCT quality across five domains: randomization, allocation concealment, blinding, incomplete outcome data, and selective reporting. Each domain assessment categorized bias potential into “low risk of bias,” “uncertain risk of bias,” and “high risk of bias.” Any discrepancies in assessments were resolved through consultation with an adjudicator (via JY L) to ensure consensus.
Data synthesis and analysis
During full-text screening, the level of agreement between reviewers was evaluated using Kappa statistics [18]. The occurrences of hypothyroidism, type 2 diabetes mellitus, hyperthyroidism, hypertriglyceridemia, hypercholesterolemia, hypoglycemia, and hyperglycemia were quantified for each study. The odds ratio (OR) and confidence intervals (CIs) were determined by the Peto approach. Statistical heterogeneity across trials was assessed using Cochrane’s Q statistic, with consistency measured using the I2 statistic. Selection between random- or fixed-effects models was guided by the observed heterogeneity. Subgroup analyses were conducted according to the type of base therapy, PARPi variants, and solid tumor types. Publication bias was assessed using Egger’s test and Begg’s test [19], accompanied by a sensitivity analysis. Data management was executed using Microsoft Office Excel 2007, while statistical analyses were primarily performed using RevMan Manager and Stata software (version 15).
Data and resource availability
The datasets generated and analyzed during the current study are accessible from the corresponding author upon reasonable request.
Results
Study selection and characteristics
Initially, 5236 studies were identified in our search, with 200 studies undergoing comprehensive evaluation (supplementary materials S1). Among these, 40 studies were conference proceedings, 127 did not investigate outcomes related to endocrine and metabolic dysfunction, and 7 were cross-sectional studies. Following rigorous review, 26 articles [12–14, 20–42] (see Table 1) were selected for analysis, with 21 studies included in the final hyperglycemia analysis. Additionally, 3 articles on hyperthyroidism, 6 on type 2 diabetes mellitus, 5 on hypercholesterolemia, 4 on hypoglycemia, 5 on hypothyroidism, and 4 on hypertriglyceridemia were deemed pertinent for inclusion in this meta-analysis based on adequacy and relevance. In total, data from 30 RCTs encompassing 9,590 patients were included. Among these patients, 3,533 (36.84%) were diagnosed with ovarian cancer, while 3,640 (37.95%) had breast cancer. The remaining patients had various types of cancer including lung, urothelial carcinoma, pancreatic, prostate, and colorectal cancer, with 10.74% (1,030 patients), 2.21% (212 patients), 2.82% (271 patients), 9.73% (934 patients), and 1.35% (130 patients), respectively (Table 1). During the full-text screening process, a substantial level of agreement (κ = 0·77) achieved among reviewers [18].
Table 1.
The characteristic of included literature
| Author, Year | ClinicalTrials number | Phase | Types of diseases | Interventions | Control | Duration (year) | Intervention | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Male | Female | Mean/median age (years) | N | Male | Female | Mean age (years) | Trial group, dose | control | |||||
| Rosenberg J E, 2023 [20] | NCT03459846 | Phase II | MUC | 78 | 56 | 22 | 79 (47–89) | 76 | 55 | 21 | 72 (45–88) | 2 years | Olaparib + durvalumab | Durvalumab + placebo |
| Rodler E, 2023 [21] | NCT02595905 | Phase II | BC | 162 | 0 | 162 | 56 (47–66; 27–80) | 158 | 1 | 157 | 56 (47–64; 26–80) | 5 years | Veliparib + cisplatin | Cisplatin + placebo |
| Kindler H L, 2023 [22] | NCT02184195 | phase III | PAAD | 92 | 53 | 39 | NA | 62 | 31 | 31 | NA | 4 years | Olaparib | Placebo |
| Fizazi K. 2023 [23] | NCT02975934 | phase III | PRAD | 270 | 270 | 0 | 70 (45–90) | 135 | 135 | 0 | 71 (47–92) | 4 years | Rucaparib | Physician’s choice control |
| Vignani F, 2023 [24] | NCT03945084 | Phase II | MUC | 39 | 29 | 10 | 71.0(64.4–76.5) | 19 | 14 | 5 | 68.4(57.5–75.4) | 3 years | Niraparib + best supportive care | Best supportive care |
| Trillsch F, 2022 [25] | NCT01874353 | phase III | OC | 218 | 0 | 218 | NA | 109 | 0 | 109 | NA | 2 years | Olaparib | Placebo |
| Saad F, 2022 [12] | NCT01972217 | phase II | PRAD | 71 | 71 | 0 | 70 (65–75) | 71 | 71 | 0 | 70 (65–75) | 1 year | Olaparib + abiraterone |
Placebo + abiraterone |
| Liu J F, 2022 [10] | NCT02446600 | Phase III | OC | 189 | 0 | 189 | NA | 187 | 0 | 187 | NA | 5 years | Olaparib | Platinum-based chemotherapy |
| Kristeleit R, 2022 [13] | NCT02855944 | phase III | OC | 214 | 0 | 214 | NA | 113 | 0 | 113 | NA | 3.5 years | Rucaparib | Chemotherapy |
| Govindan R, 2022 [28] | NCT02264990 | Phase III | NSCLC | 297 | 207 | 90 | NA | 298 | 206 | 92 | NA | 3 years | Veliparib + chemotherapy | Chemotherapy |
| Geyer C E, 2022 [29] | NCT02032823 | phase III | BC | 921 | 2 | 919 | NA | 915 | 4 | 911 | NA | 1 year | Olaparib | Placebo |
| Ledermann J A, 2020 [14] | NCT01968213 | phase III | OC | 375 | 0 | 375 | NA | 189 | 0 | 189 | NA | 1 year | Rucaparib | Placebo |
| Wu X H, 2021 [30] | NCT03705156 | phase III | OC | 177 | 0 | 177 | 53.0 (35–78) | 88 | 0 | 88 | 55.0 (38–72) | 1 year | Niraparib | Placebo |
| Chiorean E G, 2021 [31] | NCT02890355 | Phase II | PAAD | 59 | 31 | 28 | 67 (61–72) | 58 | 33 | 25 | 67 (61–71) | 3 years | Veliparib + mFOLFIRI | mFOLFIRI |
| Byers L A, 2021 [32] | NCT02289690 | Phase II | SCLC | 61 | 40 | 21 | 62.0(39.0–77.0) | 61 | 38 | 23 | 63.0 (37.0–87.0) | 1 year | Veliparib + CE | Placebo + CE |
| Ai X, 2021 [33] | NCT03516084 | Phase III | SCLC | 125 | 101 | 24 | 61.0 (8.86) | 60 | 49 | 11 | 61.5 (6.56) | 1 year | Niraparib | Placebo |
| Diéras V, 2020 [34] | NCT02163694 | phase III | BC | 337 | 4 | 333 | 47 (39–54) | 172 | 3 | 169 | 47 (39–54) | 1 year | Veliparib | Placebo |
| de Bono J, 2020 [35] | NCT02987543 | phase III | PRAD | 256 | 256 | 0 | 69 (47–91 | 131 | 131 | 0 | 69 (49–87) | 1 year | Olaparib | Enzalutamide + abiraterone |
| Owonikoko T K, 2019 [36] | NCT01642251 | Phase II | SCLC | 64 | 34 | 30 | 66 (59,72) | 64 | 32 | 32 | 64 (59,70) | 2 years | Veliparib + CE | Placebo + CE |
| Gorbunova V, 2019 [37] | NCT02305758 | phase II | CRC | 65 | 44 | 21 | 59 (26–84) | 65 | 40 | 25 | 64 (43–84) | 2 years | Veliparib | Placebo |
| Ray-Coquard I, 2019 [38] | NCT02655016 | phase III | OC | 487 | 0 | 487 | 61.0 (32.0–87.0) | 246 | 0 | 246 | 60.0 (26.0–85.0) | 2 years | Olaparib + bevacizumab | Placebo + Bevacizumab |
| Coleman R L, 2019 [39] | NCT02470585 | phase III | OC | 383 | 0 | 383 | 62 (22–88) | 375 | 0 | 375 | 62 (33–86) | 3 years | Veliparib | Placebo |
| Loibl S, 2018 [40] | NCT02032277 | phase III | BC | 316 | 0 | 316 | 51 (41–59) | 160 | 0 | 160 | 49 (40–57) | 1 year | Veliparib + CP | CP |
| Han H S, 2018 [41] | NCT01506609 | phase II | BC | 97 | 2 | 95 | 44(25–65) | 99 | 2 | 97 | 46(24–66) | 1 year | Veliparib + CP | Placebo + CP |
| Mirza M R, 2016 [42] | NCT01847274 | phase III | OC | 138 | 0 | 138 | 57 (36–83) | 65 | 0 | 65 | 58 (38–73) | 1 year | Niraparib | Placebo |
| O’Shaughnessy J, 2011 [43] | NCT00540358 | phase II | BC | 61 | 0 | 61 | 56 (34–76) | 62 | 0 | 62 | 53 (26–80) | 3 years | Iniparib + GC | GC |
Metastatic Urothelial Carcinoma: MUC; breast cancer: BC; pancreatic cancer: PAAD; Prostate Cancer: PRAD; ovarian cancer: OC; nonesmall-cell lung cancer: NSCLC; small-cell lung cancer: SCLC; colorectal cancer: CRC; cisplatin and etoposide: CE; carboplatin + paclitaxel: CP; gemcitabine–carboplatin: GC
Incidence and OR of hyperglycemia, hypoglycemia, and type 2 diabetes mellitus
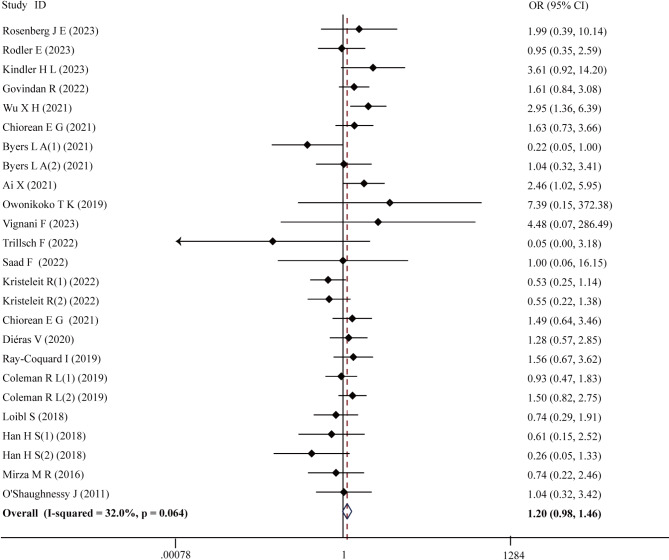
In the investigation of the incidence and OR of any grade hyperglycemia, 25 RCTs were meticulously selected. A total of 7,190 patients participated in these trials, with 4,120 patients in the intervention group and 3,070 patients in the control group. In the intervention group, any grade hyperglycemia occurred in 279 out of 4,120 patients, representing an incidence of 6.77%. In contrast, 5.92% of patients in the control groups experienced this, with 182 incidents among 3,070 patients. Notably, the use of PARPi did not demonstrate a significant increase in the OR for any grade hyperglycemia (OR = 1.20, 95% CI 0.98–1.46; P = 0.076) (Fig. 1). No significant heterogeneity was detected in this analysis (χ2 = 35.30, P = 0.064; I2 = 32.0%).
Fig. 1.
The forest plot of any grade hyperglycemia
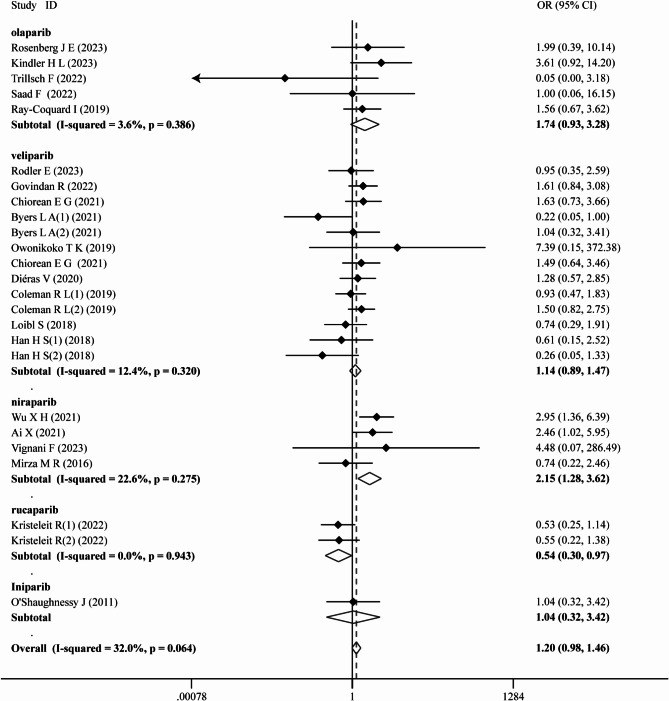
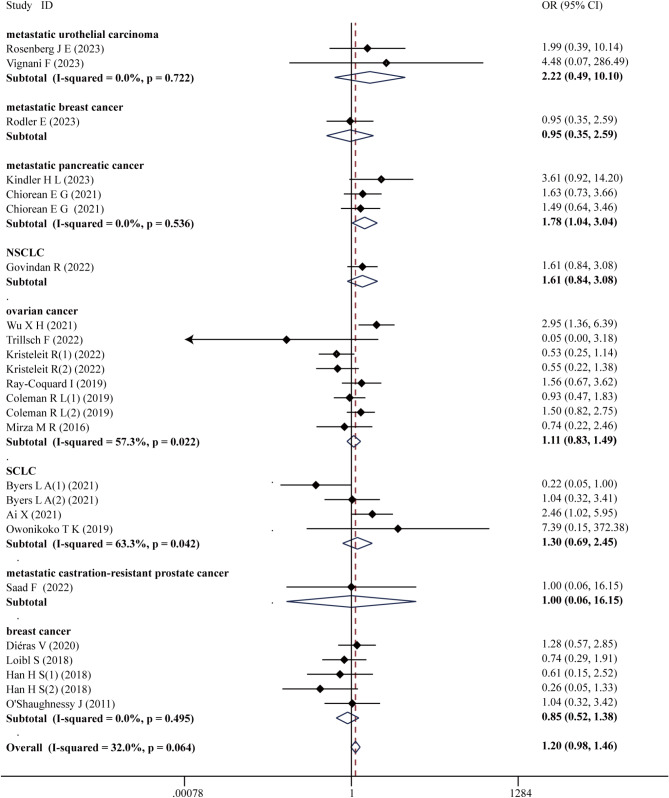
Subsequent subgroup analysis was performed based on the type of PARPi, tumor type, and the specific treatment modality provided in the intervention group (chemotherapy + PARPi, PD-L1 inhibitors + PARPi, antiVEGF + PARPi, and PARPi monotherapy). Interestingly, treatment with niraparib exhibited a notable association with an increased risk of hyperglycemia (OR = 2.15, 95% CI 1.28–3.62; P = 0.004), while rucaparib demonstrated potential in ameliorating hyperglycemia (OR = 0.54, 95% CI 0.30–0.97; P = 0.039) (Fig. 2). Notably, patients with metastatic pancreatic cancer receiving PARPi were found to be more susceptible to hyperglycemia (OR = 1.78, 95% CI 1.04–3.04; P = 0.036) (Fig. 3). Subgroup analyses by type of PARPi-based therapy unveiled no significant variations (supplementary materials S2), while sensitivity analyses confirmed result stability (supplementary materials S3). Furthermore, no evidence of publication bias was detected according to the Egger test (P = 0.322), Begg test (P = 0.661), and funnel plot (supplementary materials S4).
Fig. 2.
Subgroup analysis of any grade hyperglycemia by type of PARPi
Fig. 3.
Subgroup analysis of any grade hyperglycemia by type of tumor
Regarding serious hyperglycemia, data extracted from 2 studies involving 352 patients revealed an incidence of 0.4% among the 226 patients who received PARPi therapy. By contrast, serious hyperglycemia was reported in 0.7% of patients in the control group (1 event among 126 patients). Furthermore, PARPi therapy showed no significant increase in the risk of serious hyperglycemia (OR = 0.55, 95% CI 0.03–9.95; P = 0.688) (supplementary materials S5). This analysis showed no significant heterogeneity (χ2 = 1.79, P = 0.181; I2 = 44.2%).
The incidence and OR of hypoglycemia were assessed in four studies involving a total of 1,339 patients. Among these, 854 patients were assigned to PARPi therapy in the intervention group, whereas 485 patients were included in the control arm. The incidence of hypoglycemia was 0.5% (5 events among 854 patients) in the intervention arm and 0.4% (2 events among 485 patients) in the control arm. No significant difference in hypoglycemia incidence was observed between the intervention and control groups (OR = 1.51, 95% CI 0.32–7.05; P = 0.603) (supplementary materials S6). Heterogeneity analysis revealed no notable distinctions (χ2 = 2.66, P = 0.447; I2 = 0.0%).
In this study, we evaluated the incidence and OR of type 2 diabetes mellitus. A total of 2,916 patients were included in six studies. The incidence of type 2 diabetes mellitus among those undergoing PARPi therapy was 0.1% (4 cases among 1,701 patients). This contrasted with a 0.2% incidence observed in the control group (three cases among 1,215 patients). Our analysis revealed no significant difference in the risk of type 2 diabetes mellitus in comparison with control (OR = 0.99, 95% CI 0.22–4.54; P = 0.993) (supplementary materials S7). Furthermore, our findings indicated no heterogeneity in this analysis (χ2 = 6.93, P = 0.226; I2 = 27.8%).
Incidence and OR of thyroid function
The incidence and OR of hyperthyroidism and hypothyroidism were examined in patients undergoing PARPi treatment versus control. Three studies were analyzed for the hyperthyroidism investigation, comprising 1,275 patients in total. Among these, 641 patients received PARPi while 634 patients underwent standard treatment. Hyperthyroidism manifested in 4 out of 641 patients receiving PARPi, resulting in an incidence of 0.6%. In comparison, the control group exhibited a 0.4% incidence of hyperthyroidism, with 3 cases among 634 patients. The analysis demonstrated that PARPi did not markedly influence the likelihood of developing hyperthyroidism (OR = 1.32, 95% CI 0.30–5.85; P = 0.714) (supplementary materials S8A). Moreover, the analysis revealed no significant heterogeneity (χ2 = 2.07, P = 0.356; I2 = 3.2%).
In the investigation of hypothyroidism, five studies were meticulously examined, encompassing a total of 3,224 patients. The incidence of hypothyroidism was 0.8%, with 15 incidences among 1,752 patients, compared to 0.6% in the control group, with 10 incidences among 1,472 patients. No significant increase in hypothyroidism risk was observed in patients receiving PARPi therapy (OR = 1.34, 95% CI 0.60–3.01; P = 0.478) (supplementary materials S8B). Furthermore, this analysis revealed no noticeable heterogeneity (χ2 = 2.14, P = 0.711; I2 = 0.0%).
Incidence and OR of dyslipidemia
Dyslipidemia, comprising hypertriglyceridemia and hypercholesterolemia, was analyzed in our study. Hypertriglyceridemia was studied across four trials involving 1,671 patients. The frequency of hypertriglyceridemia was 3.33% (36 cases out of 1,066 patients), compared to 3.14% in the control group (19 cases out of 605 patients). No significant increase was observed between the intervention and control groups (OR = 1.26, 95% CI 0.71–2.23; P = 0.421) (supplementary materials S9A). Additionally, this analysis unveiled no heterogeneity of significance (χ2 = 2.14, P = 0.492; I2 = 0.0%).
For hypercholesterolemia, five studies were analyzed, involving 2,157 patients in total. Within this cohort, 1,389 patients in the intervention arm received PARPi, while 768 patients in the control arm received standard therapy. The incidence of hypercholesterolemia was 3.67% (51 events among 1,389 patients) in the intervention arm and 2.47% (19 cases among 768 patients) in the control arm. Notably, PARPi did not significantly increase the risk of hypercholesterolemia (OR = 1.62, 95% CI 0.98–2.69; P = 0.060) (supplementary materials S9B). Furthermore, this analysis did not show any significant heterogeneity (χ2 = 6.68, P = 0.154; I2 = 40.1%).
Quality of the studies
Upon assessment based on the Cochrane Collaboration guidelines, all included studies were identified to carry a high risk of bias (supplementary materials S10 and S11). Among the studies, only seven were deemed of high quality, while 14 were categorized as being at high risk of bias.
Discussion
Our study elucidates the intricate relationships between PARPi, endocrine function, and metabolic health, thereby guiding clinical applications and advancing scientific inquiry. We conducted a comprehensive analysis of 26 RCTs, including 16 phase III and 10 phase II trials, to assess the risk of endocrine and metabolic disturbances in patients with solid tumor receiving PARPi. The overall incidence of hyperglycemia was relatively low at 6.77%, it was notably less frequent compared to other adverse events such as nausea (68.8%), vomiting (47.8%), anemia (47.8%), diarrhea (25.3%), thrombocytopenia (23.0%), neutropenia (39.6%), and hypertension (12%) [43–45]. Subgroup analysis revealed a significantly elevated risk of hyperglycemia associated with niraparib treatment. This trend was similarly observed in pancreatic cancer patients. In contrast, rucaparib appeared to be associated with a potential reduction in hyperglycemia risk. Other metabolic conditions such as hypoglycemia, diabetes, thyroid dysfunction, and dyslipidemia, exhibited low incidence rates and no significant differences. To our knowledge, this is the first large-scale study demonstrating an increased risk of hyperglycemia associated with PARPi treatment compared to placebo. These findings underscore the need for vigilant monitoring of metabolic health in patients undergoing PARPi therapy.
The interaction between PARPi and glycometabolism deserves further investigation. Preclinical studies suggested that PARPi may mitigate diabetic vascular complications by reducing reactive oxygen species (ROS) and inflammatory responses [46–48]. Surprisingly, our meta-analysis indicated that niraparib, in particular, is associated with a significant increase in the risk of hyperglycemia across all grades, with a pronounced effect observed in the pancreatic cancer patient subset. Although the etiology of glucose toxicity remains incompletely understood, it seems primarily linked to the inhibition of proteins beyond the intended PARP targets, indicating off-target effects. Furthermore, increased PARP1 trapping capacity has been associated with heightened toxicity in normal tissues. In our meta-analysis, niraparib exhibited the highest PARP1 trapping ability, correlating with its increased risk of hyperglycemia compared to rucaparib, veliparib, and olaparib [9, 49, 50]. Niraparib may significantly contribute to the development of hyperglycemia by off-target inhibition of dopamine and norepinephrine transporter activity, thereby impairing their cellular uptake [51, 52]. Furthermore, premature aging and loss of genome stability may underlie the disruptions in endocrine and metabolic function [53]. This evaluation underscores the significance of comprehensively grasping PARPi mechanisms and their impact on cancer therapy and blood glucose control. The presented insights have the potential to influence forthcoming research and clinical protocols, notably in the context of patients with cancer and diabetes. The capacity of PARPi to influence glucose levels presents novel therapeutic avenues and calls for a meticulous reassessment of their safety profile, specifically concerning metabolic outcomes.
Further investigation is warranted to understand the intricate interactions between PARPi and lipid metabolism. While preclinical studies hint at a protective role of PARPi in lipid metabolism [9, 54, 55], definitive clinical validation is still pending. Our meta-analysis results intriguingly indicate that PARPi have no significant impact on lipid metabolism. Discrepancies between preclinical and clinical findings likely stem from various factors, including interspecies differences in drug metabolism, limitations of animal models in cancer research, variations in drug pharmacokinetics and pharmacodynamics across species, diverse environmental and lifestyle factors in humans, genetic predispositions, inconsistencies in clinical trial methodologies, and differences in data analysis approaches [56–58]. Integrating these factors fosters a nuanced comprehension that can facilitate the translation of preclinical insights into clinically relevant therapies.
In our investigation, we explored the influence of PARPi on thyroid function—a topic marginally explored in the scientific discourse. Our study uncovered no significant impact of PARPi on thyroid function, aligning with findings by Tian X et al. [59]. Notwithstanding, the therapeutic promise of PARPi in thyroid cancer treatment is substantial. X. Hou et al. [60] exhibited the potent anti-tumor effects of niraparib on thyroid cancer stem-like cells in vitro and in vivo. Moreover, C. Passaro et al. [61] illustrated that PARPi can enhance the therapeutic efficacy of the oncolytic adenovirus dl922-947. This strategy holds immense potential, not only for anaplastic thyroid carcinoma but also for a spectrum of cancers amenable to oncolytic virotherapy. These discoveries lay the groundwork for forthcoming research in cancer therapeutics.
Our meta-analysis, which scrutinized subgroups based on the type of PARPi-based therapy, revealed that combined treatments with PD-L1 inhibitors, chemotherapy, or anti-VEGF did not alter the risk of hyperglycemia. Contrary to expectations, PARPi monotherapy exhibited a heightened risk of hyperglycemia based on our findings (OR = 1.24, 95% CI 0.94–1.62). Owing to limited sample size, the impact of combination therapies on other metabolic conditions was not explored. Nevertheless, investigating combination therapies could be a promising avenue in future research to mitigate side effects and enhance therapeutic effectiveness.
PARPi exhibit potential risks on human metabolism and endocrine systems, underscoring the necessity for future investigations to target cancer cells more precisely. Given the established link between hyperglycemia and key stages of cancer such as tumor initiation, progression, metastasis, and therapy resistance [9, 46, 55], underscores the vital requirement for vigilant glucose monitoring in solid tumor patients undergoing PARPi treatment, especially those with pancreatic cancer or receiving niraparib [1, 3]. This observation plays a pivotal role in shaping tailored treatment approaches to improve patient outcomes by proactively managing treatment-related issues.
Our study reveals significant gaps in understanding PARP inhibitors (PARPi) effects on the endocrine and metabolic systems, necessitating further exploration [7, 62]. Key deficiencies include the lack of predictive biomarkers for patient responses and uncertainties regarding long-term systemic effects [63]. Current research efforts focus on identifying genetic and molecular biomarkers and assessing efficacy and safety. Recent advancements in PARPi include the development of more selective PARP1 inhibitors like Saruparib, showing promise in clinical trials [64]. Over the next five years, substantial progress in PARPi research is expected, with a focus on predictive biomarkers and combination therapies to balance benefits and risks in cancer management [9, 23, 24, 26]. Interdisciplinary research in pharmacology, genetics, immunology, and endocrinology is key to understanding PARPi’s effects on metabolism, guiding personalized medicine and therapeutic advances. The rise in clinical trials combining PARPi with other treatments may revolutionize oncology [25, 27, 29].
Although our analysis provided valuable insights, it was constrained by several factors. The primary studies, focused on survival outcomes with PARPi, overlooked the risks of metabolic and endocrine abnormalities, potentially contributing to their underrepresentation in the reviewed RCTs. Relying solely on published data may have led to an incomplete identification of rare or delayed adverse events. Caution is advised in interpreting our combined data due to the potential for selection bias and wide confidence intervals. The exclusion of crucial baseline risk factors such as patient comorbidities, metabolic syndrome, age, and body mass index limited the scope of our risk assessment for PARPi treatment. Insufficient long-term follow-up data hindered a comprehensive evaluation of PARPi’s chronic effects on endocrine and metabolic functions. Additionally, the generalizability of our findings may be compromised by demographic, treatment setting, and contextual variations among studies. The discussion failed to address the dose-response relationship between PARPi and disruptions in endocrine and metabolic functions, potentially restricting the clinical relevance of our results. Subsequent research should integrate these aspects to enhance the accuracy and dependability of findings in this field.
Conclusion
PARPi have been shown to adversely affect endocrine and metabolic processes in patients with solid tumors. Particular emphasis should be placed on the risk of hyperglycemia associated with niraparib, particularly in pancreatic cancer patients. It is important to recognize that the accuracy of these findings may be influenced by the limitations previously discussed. Future research should prioritize multicentric prospective observational studies and registries to enhance our understanding. Additionally, elucidating the mechanisms underlying the endocrine and metabolic effects of PARPi is essential for ensuring the reliability of these results.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors would like to acknowledge Sichuan Canc Hosp & Inst and Chengdu University of Traditional Chinese Medicine for their contribution to the literature search.
Author contributions
Shunlian Fu, Pingjin Zou, Meihua Chen, and Jinyi Lang: Conceptualization, Design, Methodology, Writing-original draft, Writing-review & editing, the acquisition, analysis, or interpretation of data. Pingjin Zou, Zengyi Fang, Xinxiang Zhou, Junyang Chen, Cuicui Gong, Li Quan, Bing Lin: Writing-original draft, Writing-review & editing, Final approval of the version to be submitted to and published in the journal. All authors approved the final manuscript and have participated sufficiently in the work to take public responsibility for appropriate portions of the content.
Funding
This work was supported by National Natural Science Foundation of China (81703070), Health Commission of Sichuan Province (2024–803), Chengdu Technology Bureau (2024-YF05-02230-SN), Sichuan Science and Technology Program (2020JDJQ0026), and Beijing Xisike Clinical Oncology Research Foundation (Y-XD202001-0024). The funding source had no involvement in the study’s design, data collection and analysis, interpretation, or the preparation and submission of the report.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Guarantor statement
Meihua Chen and Jinyi Lang are the guarantors of this work and, possessing full access to all study data and taking responsibility for the integrity and accuracy of the data analysis. All competing interests are unrelated to the submitted work.
Data sharing
Research data are securely stored and will be shared upon request to the corresponding author.
Originality and prior publication
All authors declared that the manuscript, or any part of it, has not been published nor under consideration for publication elsewhere. The study described in the manuscript has not been previously presented at any meetings, published in abstract form, or posted on a preprint server.
Declaration of generative AI in scientific writing
In our final review, we utilized AI and AI-related tools to reduce redundancy and enhance the readability and language of the article. Our aim was to produce a balanced, precise, and grammatically correct version of the original text. Throughout this process, we maintained oversight and control over the resulting text, ensuring adherence to conventional academic writing principles, such as objectivity, clarity, and appropriate grammatical structures. We also followed established style guidelines for citations and footnote formatting.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qiu Chen, Email: chenqiu1005@cdutcm.edu.cn.
Jinyi Lang, Email: langjy610@163.com.
Meihua Chen, Email: chenmeihua@scszlyy.org.cn.
References
- 1.Curtin NJ, Szabo C. Poly(ADP-ribose) polymerase inhibition: past, present and future. Nat Rev Drug Discov. 2020;19(10):711–36. [DOI] [PubMed] [Google Scholar]
- 2.Ricci AD, Rizzo A, Novelli M, Tavolari S, Palloni A, Tober N, Abbati F, Mollica V, S, Turchetti DEL. Specific toxicity of maintenance Olaparib Versus Placebo in Advanced malignancies: a systematic review and Meta-analysis. Anticancer Res. 2020;40(2):597–608. [DOI] [PubMed] [Google Scholar]
- 3.Yap TA, Sandhu SK, Carden CP, de Bono JS. Poly(ADP-ribose) polymerase (PARP) inhibitors: exploiting a synthetic lethal strategy in the clinic. CA Cancer J Clin. 2011;61(1):31–49. [DOI] [PubMed] [Google Scholar]
- 4.Di Federico A, Tateo V, Parisi C, Formica F, Carloni R, Frega G, Rizzo A, Ricci D, Di Marco M, Palloni A et al. Hacking pancreatic Cancer: Present and Future of Personalized Medicine. Pharmaceuticals (Basel). 2021; 14(7). [DOI] [PMC free article] [PubMed]
- 5.Ricci AD, Rizzo A, Brandi G. DNA damage response alterations in gastric cancer: knocking down a new wall. Future Oncol. 2021;17(8):865–8. [DOI] [PubMed] [Google Scholar]
- 6.Rizzo A, Mollica V, Merler S, Morelli F, Sorgentoni G, Oderda M, Santoni M, Massari F. Incidence of grade 3–4 adverse events, dose reduction, and treatment discontinuation in castration-resistant prostate cancer patients receiving PARP inhibitors: a meta-analysis. Expert Opin Drug Metab Toxicol. 2022;18(3):235–40. [DOI] [PubMed] [Google Scholar]
- 7.Chu YY, Yam C, Yamaguchi H, Hung MC. Biomarkers beyond BRCA: promising combinatorial treatment strategies in overcoming resistance to PARP inhibitors. J Biomed Sci. 2022;29(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosellini M, Santoni M, Mollica V, Rizzo A, Cimadamore A, Scarpelli M, Storti N, Battelli N, Montironi R, Massari F. Treating prostate Cancer by Antibody-Drug Conjugates. Int J Mol Sci. 2021; 22(4). [DOI] [PMC free article] [PubMed]
- 9.Szántó M, Gupte R, Kraus WL, Pacher P, Bai P. PARPs in lipid metabolism and related diseases. Prog Lipid Res. 2021;84:101117. [DOI] [PubMed] [Google Scholar]
- 10.Liu JF, Brady MF, Matulonis UA, Miller A, Kohn EC, Swisher EM, Cella D, Tew WP, Cloven NG, Muller CY, et al. Olaparib with or without Cediranib Versus Platinum-based chemotherapy in recurrent platinum-sensitive ovarian Cancer (NRG-GY004): a randomized, Open-Label, phase III trial. J Clin Oncol. 2022;40(19):2138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geyer CE, Garber JE, Gelber RD, Yothers G, Taboada M, Ross L, Rastogi P, Cui K, Arahmani A, Aktan G, et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Annals Oncology: Official J Eur Soc Med Oncol. 2022;33(12):1250–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saad F, Thiery-Vuillemin A, Wiechno P, Alekseev B, Sala N, Jones R, Kocak I, Chiuri VE, Jassem J, Fléchon A, et al. Patient-reported outcomes with olaparib plus abiraterone versus placebo plus abiraterone for metastatic castration-resistant prostate cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol. 2022;23(10):1297–307. [DOI] [PubMed] [Google Scholar]
- 13.Kristeleit R, Lisyanskaya A, Fedenko A, Dvorkin M, de Melo AC, Shparyk Y, Rakhmatullina I, Bondarenko I, Colombo N, Svintsitskiy V, et al. Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(4):465–78. [DOI] [PubMed] [Google Scholar]
- 14.Ledermann JA, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, Colombo N, Weberpals JI, Clamp AR, Scambia G, et al. Rucaparib for patients with platinum-sensitive, recurrent ovarian carcinoma (ARIEL3): post-progression outcomes and updated safety results from a randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(5):710–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, Tolaney SM. Incidence of endocrine dysfunction following the use of different Immune checkpoint inhibitor regimens: a systematic review and Meta-analysis. JAMA Oncol. 2018;4(2):173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed). 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zucatti KP, Teixeira PP, Wayerbacher LF, Piccoli GF, Correia PE, Fonseca NKO, Moresco KS, Guerra BA, Maduré MG, Farenzena LP, et al. Long-term effect of Lifestyle interventions on the Cardiovascular and all-cause mortality of subjects with prediabetes and Type 2 diabetes: a systematic review and Meta-analysis. Diabetes Care. 2022;45(11):2787–95. [DOI] [PubMed] [Google Scholar]
- 18.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Res ed). 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg JE, Park SH, Kozlov V, Dao TV, Castellano D, Li JR, Mukherjee SD, Howells K, Dry H, Lanasa MC, et al. Durvalumab Plus Olaparib in previously untreated, platinum-ineligible patients with metastatic urothelial carcinoma: a Multicenter, Randomized, phase II trial (BAYOU). J Clin Oncology: Official J Am Soc Clin Oncol. 2023;41(1):43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodler E, Sharma P, Barlow WE, Gralow JR, Puhalla SL, Anders CK, Goldstein L, Tripathy D, Brown-Glaberman UA, Huynh TT, et al. Cisplatin with veliparib or placebo in metastatic triple-negative breast cancer and BRCA mutation-associated breast cancer (S1416): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2023;24(2):162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kindler HL, Yoo HK, Hettle R, Cui KY, Joo S, Locker GY, Golan T. Patient-centered outcomes in the POLO study of active maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. Cancer. 2023;129(9):1411–8. [DOI] [PubMed] [Google Scholar]
- 23.Fizazi K, Piulats JM, Reaume MN, Ostler P, McDermott R, Gingerich JR, Pintus E, Sridhar SS, Bambury RM, Emmenegger U, et al. Rucaparib or Physician’s choice in metastatic prostate Cancer. N Engl J Med. 2023;388(8):719–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vignani F, Tambaro R, De Giorgi U, Giannatempo P, Bimbatti D, Carella C, Stellato M, Atzori F, Aieta M, Masini C, et al. Addition of Niraparib to best supportive care as maintenance treatment in patients with Advanced Urothelial Carcinoma whose disease did not Progress after First-line platinum-based Chemotherapy: the Meet-URO12 Randomized Phase 2 Trial. Eur Urol. 2023;83(1):82–9. [DOI] [PubMed] [Google Scholar]
- 25.Trillsch F, Mahner S, Ataseven B, Asher R, Aryal N, Dubot C, Clamp A, Penson RT, Oza A, Amit A, et al. Efficacy and safety of olaparib according to age in BRCA1/2-mutated patients with recurrent platinum-sensitive ovarian cancer: analysis of the phase III SOLO2/ENGOT-Ov21 study. Gynecol Oncol. 2022;165(1):40–8. [DOI] [PubMed] [Google Scholar]
- 26.Liu JF, Brady MF, Matulonis UA, Miller A, Kohn EC, Swisher EM, Cella D, Tew WP, Cloven NG, Muller CY, et al. Olaparib with or without Cediranib Versus Platinum-based chemotherapy in recurrent platinum-sensitive ovarian Cancer (NRG-GY004): a randomized, Open-Label, phase III trial. J Clin Oncology: Official J Am Soc Clin Oncol. 2022;40(19):2138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Govindan R, Lind M, Insa A, Khan SA, Uskov D, Tafreshi A, Guclu S, Bar J, Kato T, Lee KH, et al. Veliparib Plus Carboplatin and Paclitaxel Versus Investigator’s choice of standard chemotherapy in patients with Advanced Non-squamous Non-small Cell Lung Cancer. Clin Lung Cancer. 2022;23(3):214–25. [DOI] [PubMed] [Google Scholar]
- 28.Geyer CE Jr., Garber JE, Gelber RD, Yothers G, Taboada M, Ross L, Rastogi P, Cui K, Arahmani A, Aktan G, et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann Oncol. 2022;33(12):1250–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu XH, Zhu JQ, Yin RT, Yang JX, Liu JH, Wang J, Wu LY, Liu ZL, Gao YN, Wang DB, et al. Niraparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer using an individualized starting dose (NORA): a randomized, double-blind, placebo-controlled phase III trial(☆). Ann Oncol. 2021;32(4):512–21. [DOI] [PubMed] [Google Scholar]
- 30.Chiorean EG, Guthrie KA, Philip PA, Swisher EM, Jalikis F, Pishvaian MJ, Berlin J, Noel MS, Suga JM, Garrido-Laguna I, et al. Randomized Phase II study of PARP inhibitor ABT-888 (Veliparib) with modified FOLFIRI versus FOLFIRI as second-line treatment of metastatic pancreatic Cancer: SWOG S1513. Clin Cancer Res. 2021;27(23):6314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Byers LA, Bentsion D, Gans S, Penkov K, Son C, Sibille A, Owonikoko TK, Groen HJM, Gay CM, Fujimoto J, et al. Veliparib in Combination with Carboplatin and Etoposide in patients with Treatment-Naïve extensive-stage small cell Lung Cancer: a phase 2 Randomized Study. Clin Cancer Res. 2021;27(14):3884–95. [DOI] [PubMed] [Google Scholar]
- 32.Ai X, Pan Y, Shi J, Yang N, Liu C, Zhou J, Zhang X, Dong X, He J, Li X, et al. Efficacy and safety of Niraparib as maintenance treatment in patients with extensive-stage SCLC after First-Line chemotherapy: a Randomized, Double-Blind, phase 3 study. J Thorac Oncol. 2021;16(8):1403–14. [DOI] [PubMed] [Google Scholar]
- 33.Diéras V, Han HS, Kaufman B, Wildiers H, Friedlander M, Ayoub JP, Puhalla SL, Bondarenko I, Campone M, Jakobsen EH, et al. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(10):1269–82. [DOI] [PubMed] [Google Scholar]
- 34.de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, et al. Olaparib for metastatic castration-resistant prostate Cancer. N Engl J Med. 2020;382(22):2091–102. [DOI] [PubMed] [Google Scholar]
- 35.Owonikoko TK, Dahlberg SE, Sica GL, Wagner LI, Wade JL 3rd, Srkalovic G, Lash BW, Leach JW, Leal TB, Aggarwal C, et al. Randomized Phase II Trial of Cisplatin and Etoposide in Combination with Veliparib or Placebo for extensive-stage small-cell Lung Cancer: ECOG-ACRIN 2511 study. J Clin Oncology: Official J Am Soc Clin Oncol. 2019;37(3):222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorbunova V, Beck JT, Hofheinz RD, Garcia-Alfonso P, Nechaeva M, Cubillo Gracian A, Mangel L, Elez Fernandez E, Deming DA, Ramanathan RK, et al. A phase 2 randomised study of veliparib plus FOLFIRI ± bevacizumab versus placebo plus FOLFIRI ± bevacizumab in metastatic colorectal cancer. Br J Cancer. 2019;120(2):183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, Berger R, Fujiwara K, Vergote I, Colombo N, Mäenpää J, et al. Olaparib plus Bevacizumab as First-Line maintenance in Ovarian Cancer. N Engl J Med. 2019;381(25):2416–28. [DOI] [PubMed] [Google Scholar]
- 38.Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, Okamoto A, Moore KN, Efrat Ben-Baruch N, Werner TL, et al. Veliparib with First-Line Chemotherapy and as maintenance therapy in Ovarian Cancer. N Engl J Med. 2019;381(25):2403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loibl S, O’Shaughnessy J, Untch M, Sikov WM, Rugo HS, McKee MD, Huober J, Golshan M, von Minckwitz G, Maag D, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol. 2018;19(4):497–509. [DOI] [PubMed] [Google Scholar]
- 40.Han HS, Diéras V, Robson M, Palácová M, Marcom PK, Jager A, Bondarenko I, Citrin D, Campone M, Telli ML, et al. Veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in patients with BRCA1/2 locally recurrent/metastatic breast cancer: randomized phase II study. Ann Oncol. 2018;29(1):154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I, et al. Niraparib Maintenance Therapy in Platinum-Sensitive, recurrent ovarian Cancer. N Engl J Med. 2016;375(22):2154–64. [DOI] [PubMed] [Google Scholar]
- 42.O’Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, Koo IC, Sherman BM, Bradley C. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364(3):205–14. [DOI] [PubMed] [Google Scholar]
- 43.Chen X, Wen Q, Kou L, Xie X, Li J, Li Y. Incidence and risk of hypertension associated with PARP inhibitors in cancer patients: a systematic review and meta-analysis. BMC Cancer. 2023;23(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Meng J, Wang G. Risk of selected gastrointestinal toxicities associated with poly (ADP-ribose) polymerase (PARP) inhibitors in the treatment of ovarian cancer: a meta-analysis of published trials. Drug Des Devel Ther. 2018;12:3013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang C, Li J. Haematologic toxicities with PARP inhibitors in cancer patients: an up-to-date meta-analysis of 29 randomized controlled trials. J Clin Pharm Ther. 2021;46(3):571–84. [DOI] [PubMed] [Google Scholar]
- 46.Li S, Deng J, Sun D, Chen S, Yao X, Wang N, Zhang J, Gu Q, Zhang S, Wang J, et al. FBXW7 alleviates hyperglycemia-induced endothelial oxidative stress injury via ROS and PARP inhibition. Redox Biol. 2022;58:102530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szabó C, Biser A, Benko R, Böttinger E, Suszták K. Poly(ADP-ribose) polymerase inhibitors ameliorate nephropathy of type 2 diabetic Leprdb/db mice. Diabetes. 2006;55(11):3004–12. [DOI] [PubMed] [Google Scholar]
- 48.Zakaria EM, El-Bassossy HM, El-Maraghy NN, Ahmed AF, Ali AA. PARP-1 inhibition alleviates diabetic cardiac complications in experimental animals. Eur J Pharmacol. 2016;791:444–54. [DOI] [PubMed] [Google Scholar]
- 49.Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Sci (New York NY). 2017;355(6330):1152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pommier Y, O’Connor MJ, de Bono J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci Transl Med. 2016;8(362):362ps317. [DOI] [PubMed] [Google Scholar]
- 51.LaFargue CJ, Dal Molin GZ, Sood AK, Coleman RL. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019;20(1):e15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palazzo A, Ciccarese C, Iacovelli R, Cannizzaro MC, Stefani A, Salvatore L, Bria E, Tortora G. Major adverse cardiac events and cardiovascular toxicity with PARP inhibitors-based therapy for solid tumors: a systematic review and safety meta-analysis. ESMO Open. 2023;8(2):101154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li F, Drel VR, Szabó C, Stevens MJ, Obrosova IG. Low-dose poly(ADP-ribose) polymerase inhibitor-containing combination therapies reverse early peripheral diabetic neuropathy. Diabetes. 2005;54(5):1514–22. [DOI] [PubMed] [Google Scholar]
- 54.Ribeiro CF, Rodrigues S, Bastos DC, Fanelli GN, Pakula H, Foiani M, Zadra G, Loda M. Blocking lipid synthesis induces DNA damage in prostate cancer and increases cell death caused by PARP inhibition. Sci Signal. 2024; 17(831). [DOI] [PMC free article] [PubMed]
- 55.Mukhopadhyay P, Horváth B, Rajesh M, Varga ZV, Gariani K, Ryu D, Cao Z, Holovac E, Park O, Zhou Z, et al. PARP inhibition protects against alcoholic and non-alcoholic steatohepatitis. J Hepatol. 2017;66(3):589–600. [DOI] [PubMed] [Google Scholar]
- 56.Sangild PT, Shen RL, Pontoppidan P, Rathe M. Animal models of chemotherapy-induced mucositis: translational relevance and challenges. Am J Physiol Gastrointest Liver Physiol. 2018;314(2):G231–46. [DOI] [PubMed] [Google Scholar]
- 57.McClellan J, King MC. Genetic heterogeneity in human disease. Cell. 2010;141(2):210–7. [DOI] [PubMed] [Google Scholar]
- 58.Perel P, Roberts I, Sena E, Wheble P, Briscoe C, Sandercock P, Macleod M, Mignini LE, Jayaram P, Khan KS. Comparison of treatment effects between animal experiments and clinical trials: systematic review. BMJ. 2007;334(7586):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian X, Chen L, Gai D, He S, Jiang X, Zhang N. Adverse event profiles of PARP inhibitors: analysis of spontaneous reports submitted to FAERS. Front Pharmacol. 2022;13:851246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hou X, Tian M, Ning J, Wang Z, Guo F, Zhang W, Hu L, Wei S, Hu C, Yun X, et al. PARP inhibitor shuts down the global translation of thyroid cancer through promoting Pol II binding to DIMT1 pause. Int J Biol Sci. 2023;19(12):3970–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Passaro C, Volpe M, Botta G, Scamardella E, Perruolo G, Gillespie D, Libertini S, Portella G. PARP inhibitor olaparib increases the oncolytic activity of dl922-947 in in vitro and in vivo model of anaplastic thyroid carcinoma. Mol Oncol. 2015;9(1):78–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Groelly FJ, Fawkes M, Dagg RA, Blackford AN, Tarsounas M. Targeting DNA damage response pathways in cancer. Nat Rev Cancer. 2023;23(2):78–94. [DOI] [PubMed] [Google Scholar]
- 63.Zhang X, Huo X, Guo H, Xue L. Combined inhibition of PARP and EZH2 for cancer treatment: current status, opportunities, and challenges. Front Pharmacol. 2022;13:965244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Illuzzi G, Staniszewska AD, Gill SJ, Pike A, McWilliams L, Critchlow SE, Cronin A, Fawell S, Hawthorne G, Jamal K, et al. Preclinical characterization of AZD5305, a Next-Generation, highly selective PARP1 inhibitor and trapper. Clin Cancer Res. 2022;28(21):4724–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.