Abstract
Background
Syzygium guineense (Wild.) DC. is a wild indigenous tree widely used as a traditional medicine for various human ailments in Ethiopia. The purpose of this study was to quantify total phenolic (TPC) and total flavonoid (TFC) contents and determine the antioxidant and antibacterial activities of various solvent extracts of the bark of the plant.
Methods
The TPC and TFC were determined using Folin-Ciocalteu and aluminum chloride methods, respectively. The 2, 2-diphenyl-1–picrylhydrazyl (DPPH) scavenging, ferric-reducing power, and total antioxidant capacity assays were used to evaluate the antioxidant activities. Antibacterial properties were determined using the disc-diffusion and broth dilution assays.
Results
The ethanol extract of the bark was found to have high TPC (37.80 ± 3.70 mgGAE/g) and TFC (19.22 ± 1.44 mgQE/g). Similarly, the ethanol extract showed stronger DPPH scavenging activity (EC50 = 5.62 μg/mL). The ferric-reducing power and total antioxidant capacity were also strong (163.08±11.67 mgAAE/g and 143.72±2.86 mgBHTE/g of dried extract of 1 mg/mL, respectively). The lowest MIC was observed in acetone extract against S. aureus (1.56 mg/mL) and in ethanol extract against K. pneumoniae (1.56 mg/mL).
Conclusion
The ethanol extract of the bark of S. guineense possesses high TPC and TFC. In addition, it showed strong ferric-reducing power and total antioxidant capacity, asserting high antioxidant content. The extracts have shown antibacterial activities against both Gram-positive (S. aureus) and Gram-negative bacterial species. Thus, further in-depth investigations may warrant the isolation of powerful antioxidants and potent antimicrobial agents from the plant.
Keywords: Antimicrobial agents, Ethiopia, Free radical scavenging activity, Medicinal plants, Phytochemicals
Introduction
The use of medicinal plants as a fundamental component of the African traditional healthcare system is the oldest with a long track record and is widely acknowledged in all therapeutic systems. In many parts of rural Africa, traditional healers prescribing medicinal plants are the most easily accessible and affordable health resource available to the local community and at times the only treatment modality that exists [1]. Traditional medicine (TM) in Africa is holistic involving both the body and the mind and traditional healers offer information, counseling, and treatment to patients and their families in a personal manner [1]. Patients’ preferences, the low ratio of medical doctors to the total population, and the lack of effective modern medical treatment for some ailments in Africa are additional factors for the wider practice of TMs.
Ethiopia is rich in plant biodiversity and many of them are used as traditional medicinal plants. It is, therefore, imperative to assume that some of these plants have chemical compounds of therapeutic value that may be used in the treatment of major diseases such as malaria, cancer, and pathogenic microorganisms [2, 3]. According to the World Health Organization (WHO), 70–80% of Africans today depend either totally or partially on TM [4]. According to a recent report, 65% of the Ethiopian population also relies on TM and a significant proportion of the preparations are made from plants [5, 6]. The majority of Ethiopians who rely on TM are rural population and a segment of the urban population where there is little or no access to modern health care [7].
Highly diverse species of plants are used in Ethiopian TM practices and one of them is Syzygium guineense (Wild.) DC. Syzygium guineense (Family Myrtaceae) named “Duuwancho” in Sidamu-Afoo is a wild tree with edible fruits. Its distribution ranges from tropical Africa, sub-tropical and tropical Asia to Australia [8]. Traditionally, the bark of the plant is pounded, macerated, and drunk for the treatment of a health condition locally referred to as ‘ARRISHSHO’ and it cures after inducing diarrhea. ‘ARRISHSHO’ is an acute ailment characterized by pain in the hip, irritation during urination, sweating, and loss of appetite as described by the traditional healers [3]. The ripened fruits of the plant are also eaten in small amounts to treat obesity [3]. Traditionally, it is also used for the treatment of inflammation, various allergic disorders, bronchitis, dysentery, and ulcers [8]. Two bioactive 3-β-hydroxylupane-type isoprenoids, betulinic acid methylene diol ester, were isolated from the stem bark of the plant and were reported to have anti-tuberculosis activity [9]. The crude leaf extract of the plant was also reported to have in vivo antimalarial activities [10]. Various studies reported the bioactivities of S. guineense extracts including antioxidant [11], anti-diabetic [12], antibacterial [13], antihelmintic activities [14], analgesic and anti-inflammatory [15], antidiarrheal, antifungal, antiviral and anticancer activities among others [16]. Therefore, the main purpose of this study was to evaluate the total phenolic and flavonoid contents and antioxidant and antibacterial activities of various solvent extracts of the bark of this widely used traditional medicinal plant.
Materials and methods
Sample preparation and extraction
After permission was obtained from the local authorities, the specimen of S. guineense was collected from the wild habitat of Dalle district, 47 km away from Hawassa, the regional capital city of the Sidama National Regional State, in February 2022. The formal identification of the plant material was done by Melaku Wondafrash, a seasoned plant taxonomist, given the voucher specimen identification number: NT061 and is deposited in publicly available National Herbarium (ETH), Addis Ababa University, Addis Ababa, Ethiopia [17]. After the bark of the plant dried and pounded, chloroform, ethyl acetate, water, acetone, and ethanol extracts all were prepared by dissolving 10 g of the air-dried bark fine powder separately in 100 mL of each solvent. All extractions were conducted in triplicate. After keeping in an orbital shaker (Buchi, Switzerland) for 8 h at room temperature, the extract was filtered using Whatman № 1 filter paper (Whatman LTD, England) and evaporated to dryness under vacuum at 45 ºC under reduced pressure using a rotary evaporator (Buchi, 3000 series, Switzerland). Finally, the dried extracts were stored in a sealed plastic container at 4 ºC until used for the experiments.
Phytochemical analysis
Total phenolic content
The total phenolic content (TPC) was estimated by the Folin-Ciocalteu method with slight modifications [18]. A 0.1 mL of the extract (1 mg/mL) was mixed with 1 mL of diluted Folin-Ciocalteu reagent. The mixture was left for 5 min and then 1mL of sodium carbonate (7.5 g/100mL solution) was added. After incubating at 25 ºC for 90 min, the absorbance of the resulting blue color was measured at 765 nm using a UV-visible spectrophotometer (Spectronic 20, UK). The TPC was expressed in terms of milligram gallic acid equivalent per gram of dried extract (mgGAE/g) using a gallic acid calibration curve (y = 0.024x – 0.014, R2 = 0.996), and the results were calculated using the equation:
 |
Where; C = TPC (mgGAE/g), c = the concentration established from the gallic acid calibration curve (μg/mL), V = volume of extract solution in milliliter, m = weight of dried extract in gram.
Total flavonoid content
The total flavonoid content (TFC) was determined as described by Ayoola et al. [19]. , with minor modifications. The quantification was based on the formation of the yellow color of the flavonoid-aluminum complex. Briefly, a volume of 2 mL of 2% aluminum chloride was mixed with the same volume of the extracts (1 mg/mL). Then Absorbance reading at 415 nm was carried out after 1 h of incubation at room temperature against a blank sample (2 mL of sample solution and 2 mL of ethanol without aluminum chloride). The total flavonoid content was determined using a standard curve of quercetin and values were calculated as milligrams of quercetin equivalent per gram of dried extract (mgQE/g) based on the quercetin calibration curve (y = 0.011x + 0.132, R2 = 0.97) and the results were calculated using the equation:
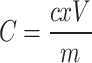 |
Where; C = TFC (mgQE/g), c = the concentration established from the quercetin calibration curve (μg/mL), V = the volume of extract solution in milliliter, m = the weight of the dried extract in gram.
DPPH scavenging activity
The DPPH scavenging activity of the extracts and references was determined as described by Engida et al. [18], . Various concentrations ranging from 10 up to 100 μg/mL) of the extracts and commercial antioxidant (ascorbic acid) were taken in different test tubes. The volume of 2 mL of freshly prepared DPPH solution (0.06% w/v) prepared in ethanol was added to each of the test tubes containing 1 mL of the extract. The extract mixtures and the reference standards (ascorbic acid and BHT) were vortexed and left to stand at room temperature in the dark for 30 min. The absorbance of the resulting solution was then measured at 517 nm. Ethanol was used as a blank. The ability to scavenge the DPPH radical was calculated as:
 |
Where Ac is the absorbance of the control and As is the absorbance in the presence of a sample of the extract.
The IC50 value is defined as the effective concentration (μg/mL) of extracts that scavenges the DPPH radical by 50%. All the tests were performed in triplicate and the graph was plotted with the average of three observations. Using the dose-response curve the IC50 values of the commercial antioxidant and crude extracts were calculated.
Ferric-reducing antioxidant power (FRAP)
The presence of antioxidants in the extract causes the reduction of the yellow ferric/ferric cyanide complex to the ferrous form which can be monitored by measuring the formation of Perl’s Prussian blue at 700 nm and was determined as described in Abebie et al. [20]. , .
Different concentrations (0.5 mg/mL and 1 mg/mL) of the bark extract of S. guineense in ethanol were mixed with 2.5 mL potassium phosphate buffer (0.2 M, PH 6.6) and 2.5 mL of 1% potassium ferricyanide. Then, the mixture was incubated at 50 0C for 20 min, and then 2.5 mL of 10% trichloroacetic acid was added to the mixture. Finally, 2.5 mL of the supernatant solution was mixed with 2.5 mL of distilled water and 0.5 mL FeCl3 (0.1%). Absorbance was measured to determine the amount of ferrous cyanide (Prussian blue) at 700 nm against a black in a UV/vis spectrophotometer. An increase in the absorbance of the reaction indicates the reducing power of the samples. The reducing power was expressed as milligram ascorbic acid equivalents per g of the extract (mg AAE/g) using the following equation (y = 0.0049x + 0.231, R2 = 0.98) based on the ascorbic acid calibration curve.
Total antioxidant using phosphomolybdenum assay
The total antioxidant capacity (TAC) was evaluated as described earlier [18] with slight modifications. The solution of different concentrations of 0.3 mL (5 and 10 mg/mL) in ethanol was prepared in different test tubes. The reagent solution was prepared by mixing 10 mL of 0.6 M sulphuric acid, 10 mL of 28 mM sodium phosphate, and 10 mL of 4 mM ammonium molybdate into a beaker, and 3 mL reagent solution was added to all the tubes. A volume of 0.3 mL of ethanol served as a blank. All the tubes were incubated at 95 0C for 90 min. The tubes were measured at 695 nm using UV-visible spectrophotometers. The TAC was expressed as a milligram of Butylated hydroxytoluene equivalent per gram of the dried extract (mg BHTE/g) based on the calibration curve (y = 0.01x + 0.15, R2 = 0.99).
The test microorganisms
Gram-negative bacteria (Escherichia coli (ATCC-25922), Salmonella typhimurium (ATCC-14028), Klebsiella pneumoniae (ATCC-13883), Pseudomonas aeruginosa (ATCC-43495)) and Gram-positive bacteria Staphylococcus aureus (ATCC-25923) were obtained from former Southern Nations, Nationalities and Peoples’ Region (SNNPR) Health Bureau Public Health Institute, Hawassa, Ethiopia in September 2022. Each microbial culture was maintained by sub-culturing on the appropriate nutrient medium and stored at 4 ºC until use [21].
The growth media preparation
The medium was prepared according to the manufacturer’s instructions. Briefly, 38 g Mueller Hinton Agar was added to a flask containing 1000 ml of distilled water and gently heated until the medium was completely dissolved. The medium was sterilized by autoclaving at 121 °C for 15 min. After cooling to about 50 ºC, approximately 15 ml of the sterilized medium was aseptically poured into 90 mm diameter sterilized Petri dishes and allowed to cool. The sterility of the prepared media was checked by incubation of blindly selected plates at 37 °C for 24 h. All chemicals, reagents, standards, and media used for the experiments were purchased from Sigma-Aldrich (St. Louise, MO, USA).
The disc diffusion assay
The disc diffusion susceptibility test was carried out as described earlier [21]. Briefly, the discs of a 6 mm diameter were prepared from Whatman № 1 filter paper using a cleaned paper puncher and were sterilized by autoclaving and dried in an oven at 120 ºC. Then, the discs were placed in a container and stored at 4 ºC until further use.
To determine the inhibition zone, the sterile discs were soaked aseptically by applying 10 μL of the crude extract at concentrations of 100 mg/mL using a sterile micropipette and then allowed to dry at room temperature for 15 min. Plates with gentamycin (10 μg) served as the positive control and discs with distilled water without the plant extracts were used as a negative control. After inoculation, for each test bacteria, the previously soaked disc with the plant extracts was placed at the center of each of the inoculated plates. The antibacterial activities of the plant extract were evaluated by measuring the diameter of the inhibition zone in each of the plates at the end of the incubation period. The diameter of the inhibition zone including the diameter of the disc was measured using sliding digital micro caliper [21].
The preparation of bacterial inoculum
The disk diffusion method was performed according to the standard procedures stated in [21]. Three to five colonies from pure cultures of each of the selected microbe species were transferred with the help of a sterile wire loop into a separately labeled test tube containing 5 mL of nutrient broth and incubated to grow at a temperature of 37 0C for 2 h. The turbidity of the actively growing broth culture was adjusted by adding sterile nutrient broth to obtain turbidity that was comparable to the 0.5 McFarland turbidity standards [22]. The microbial suspension prepared was used to inoculate the sterile MHA plates by streaking with a sterile cotton swab all over the plate. Streaking was repeated by rotating the plate approximately 60 degrees each time to ensure an even distribution of the inoculum. All of the tests were conducted in triplicate and the averages of the three measurements were used to present the results. All the plates were incubated at 37 0C for 24 h.
Determination of minimum inhibitory concentration
The minimum inhibitory concentration (MIC) was determined using the broth dilution test tube method as described earlier [21]. A total of 100 μL of pure nutrient broth was added to each test tube. The total of 10μL stock solution of 100 mg/mL extract dissolved in distilled water was subjected to two-fold serial dilutions ranging between 50 mg/mL to 1.6 mg/mL. Again, 10μL of 0.5 McFarland bacterial suspensions were added to each test tube containing 100 μL pure nutrient and 10 μL plant extracts and then the tubes were incubated at 37 0C for 24 h. After 24 h incubation, the solution was further inoculated in agar plates and incubated. The MIC was taken as the highest dilution of the extract which inhibited the growth of the bacteria. The lowest concentrations of the extracts that inhibited the bacterial growth after 24 h of incubation at 37 0C were recorded as the MIC.
Statistical analysis
The data were subjected to analysis of variance (ANOVA) using SPSS version 20 (IBM SPSS Inc. Chicago, USA). Linear regression analysis was used to calculate the EC50 value. Duncan’s multiple range tests were used to assess mean separation and the statistical significance was considered at a level of P < 0.05.
Results
The total phenolic content and total flavonoid content
The TPC of the bark of S. guineense determined by the Folin–Ciocalteu method using gallic acid as the standard showed that the ethanol fraction had a higher TPC of 37.80 ± 3.70 mgGAE/g. Meanwhile, the highest TFC value was obtained for the ethanol followed by acetone extract (19.22 ± 1.44 and 11.70± 1.11, respectively) (Table 1). Generally, the TPC and TFC contents decreased in the order of ethanol > acetone > water > ethyl acetate > chloroform.
Table 1.
Total phenolic and flavonoid contents of various solvent extracts from the bark extract of S. guineense
| Extract | TPC (mgGAE/g) | TFC (mgQE/g) |
|---|---|---|
| Chloroform | 10.30± 1.23a | 1.88± 0.64a |
| Ethyl acetate | 12.04± 1.10a | 4.12± 0.80b |
| Water | 16.61± 0.54b | 5.38± 0.66b |
| Acetone | 25.56±2.26c | 11.70±1.11c |
| Ethanol | 37.80± 3.70d | 19.22±1.44d |
Values are expressed as mean ± SD (n = 3) from triplicate experiments. Means with the same letter in a column were not significantly different at the level of p > 0.05
DPPH scavenging activity
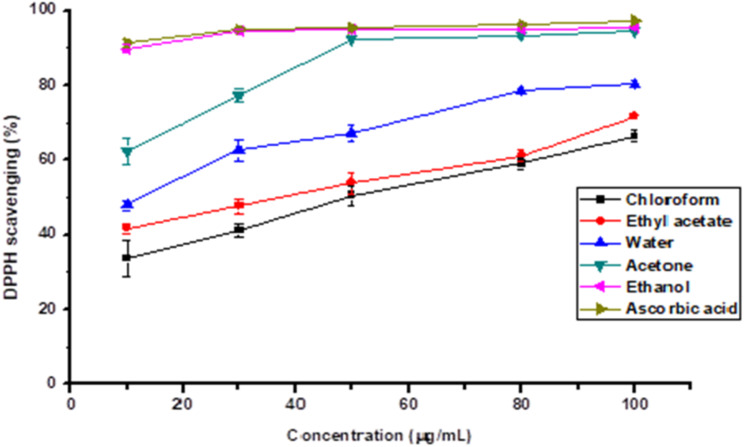
DPPH has a deep purple color in solution and changes to yellow when it encounters a proton-donating substance such as an antioxidant and a radical species which can be quantified by its absorbance reduction at wavelength 520 nm [23]. As shown in Fig. 1, the DPPH scavenging (%) effect of samples increased when the concentration of the extract increased. At 1000 μg/mL, DPPH free radical scavenging activities of the extract and ascorbic acid increased in the following order: chloroform (66.42 ± 1.44%) < ethyl acetate (71.82 ± 0.74%) < water (80.26 ± 0.86%) < acetone (94.50 ± 0.10% ) < ethanol (97.30 ± 0.73% ) < ascorbic acid (98.58 ± 0.20%). This implied that the S. guineense extracts contained a compound that can donate electron/hydrogen easily and stabilize free radicals.
Fig. 1.
DPPH radical scavenging activity (%) of various solvent extracts from dried bark of S. guineense and control (L-ascorbic acid) at different concentrations (μg/mL). The values are the average of triplicate measurements (mean ± SD)
In simple terms, the scavenging activities of the bark extracts that correspond to their concentrations, inhibiting 50% of the DPPH radicals were reported. In the present study, it was found that the ethanol extract resulted in the lowest EC50 value of 5.62 μg/mL which is very close to the positive control, ascorbic acid (5.27 μg/mL) (Table 2; Fig. 1).
Table 2.
The EC50 (μg/mL) values of DPPH scavenging activity of the bark extracts of S. guineense with various solvents
| Extract | EC50 (μg/mL) |
|---|---|
| Chloroform | 50.39 ± 5.04c |
| Ethyl acetate | 38.90 ± 7.26b |
| Water | 12.90 ± 0.82a |
| Acetone | 8.04 ± 0.47a |
| Ethanol | 5.62 ± 0.08a |
| Ascorbic acid | 5.27 ± 0.10a |
Ferric-reducing antioxidant power (FRAP)
The ethanol extract of the bark of the S. guineense was found to have strong ferric-reducing antioxidant power (163.08 ± 11.67 mgAAE/g, treatment dose = 1 mg/mL) increased in a concentration-dependent manner whereas the more nonpolar solvent, chloroform fraction, was found to have weak ferric-reducing antioxidant power (Table 3; Fig. 2A).
Table 3.
The Ferric-reducing power and total antioxidant capacity of various solvent extracts of the bark of S. guineense
| Extract | Ferric-reducing power | Total antioxidant capacity | ||
|---|---|---|---|---|
|
mgAAE/g (at 0.5 mg/mL) |
mgAAE/g (at 1 mg/mL) |
mgBHTE (at 0.5 mg/mL) |
mgBHTE/g (at 1 mg/mL) |
|
| Chloroform | 18.54 ± 0.50a | 54.22 ± 2.40a | 37.16 ± 1.26a | 62.56 ± 2.47a |
| Ethyl acetate | 38.78 ± 2.91b | 63.73 ± 3.26a | 44.94 ± 1.67ab | 69.97 ± 2.88a |
| Water | 30.16 ± 2.80ab | 55.31 ± 5.13a | 38.42 ± 1.22b | 63.80 ± 1.23b |
| Acetone | 62.78 ± 1.14c | 134.06 ± 5.13b | 51.50 ± 2.07c | 112.17 ± 5.34c |
| Ethanol | 82.83 ± 6.60d | 163.08 ± 11.67c | 80.63 ± 1.60d | 143.72 ± 2.86d |
Fig. 2.
Ferric-reducing power (mgAAE/g) (A), and total antioxidant capacity (mgBHTE/g) (B) of various solvent extracts from dried bark of S. guineense. The values are the average of triplicate measurements (mean ± SD). The values within the same concentration with the same letter in the histogram bar were not significantly different at P > 0.05
Total antioxidant capacity (TAC)
It was determined that the ethanol extract of the bark of the S. guineense was found to have strong TAC (143.72 ± 2.86 mgBHTE/g, treatment dose = 1 mg/mL) increased in a concentration-dependent manner whereas the more nonpolar solvent, chloroform fraction, was found to have less TAC in a similar trend as that of the ferric-reducing antioxidant power (Tables 3 and Fig. 2B).
Antibacterial activities
Different solvent extracts of the bark of S. guineense were tested against selected bacteria strains (S. aureus, S. typhimurium, K. pneumoniae, and E. coli) for their antibacterial activities. The inhibition zones were compared with gentamicin 10 μg which was used as positive control.
The study revealed that all bacteria strains were inhibited by the bark extracts of S. guineense with different degrees of inhibition zone (range: 25.39 ± 0.8 mm to 8.49 ± 0.26 mm in diameter). As the result indicates, the highest zone of inhibition was recorded against S. aurues with the acetone extract (25.39 ± 0.80) (Table 4; Fig. 3).
Table 4.
Inhibition zones (mm) of various solvent extracts of the bark of S. guineense
| Solvents | S. aureus | S. typhimurium | K. pneumoniae | E. coli |
|---|---|---|---|---|
| Chloroform | - | - | - | - |
| Ethyl acetate | 14.24± 0.60aC | 12.72 ± 2.36aB | 15.60 ±1.38aC | 8.49 ± 0.38aA |
| Water | 22.24 ± 1.30bC | 23.87 ± 0.21bC | 16.64 ± 0.92abB | 8.98± 0.95aA |
| Acetone | 25.39 ± 0.80cC | 23.32 ± 0.52bC | 18.26 ± 0.90bB | 11.54 ± 0.98bA |
| Ethanol | 23.35 ±1.84bcB | 21.76 ± 0.23bB | 24.39 ± 0.60cB | 8.49 ± 0.26aA |
| Gentamicin 10 μg | 24.08 ± 1.42cA | 26.88 ± 1.08cB | 29.06 ± 1.41dC | 25.63 ± 0.84cA |
The values are expressed as mean ± SD from triplicate experiments. Means with the same letter in a column (lower case) and in a raw (upper case) were not significantly different at P > 0.05
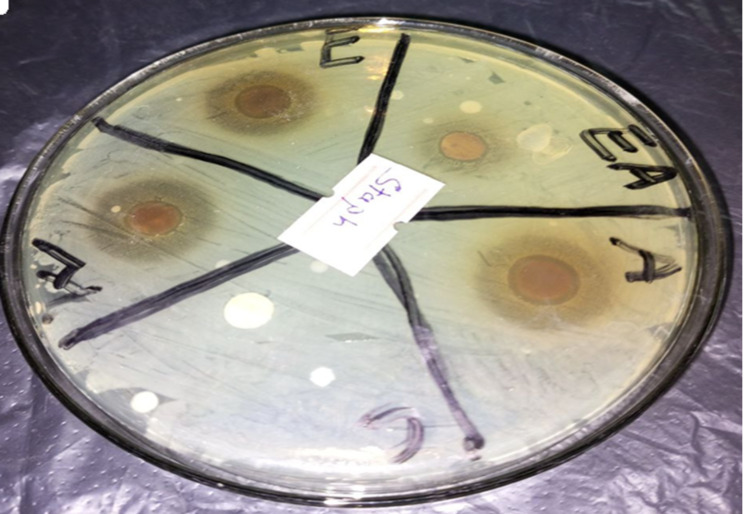
Fig. 3.
Zone of inhibitions of the bark extracts of S. guineense in comparison with gentamicin (positive control) against S. aureus. Inhibition zone of the bark extracts of S. guineense with acetone – A; ethyl acetate – EA; ethanol – E; water –W; and chloroform – C extracts
In addition to the inhibition zone determination, the MIC was determined for five extracts of the bark of the plant against four different bacterial strains. Staphylococcus aureus was relatively the most sensitive strain against acetone and ethanol extracts of the bark of S. guineense with MIC values of 1.6 mg/mL and 3.12 mg/mL, respectively. Klebsiella pneumonia was also found to be highly sensitive to the ethanol extracts of the bark of S. guineense (MIC = 1.56 mg/mL). The bacterial strain E. coli was found to be less sensitive even at higher doses of 50 mg/mL of solvent extracts (Table 5; Fig. 4). Gentamicin was used as a positive control and completely inhibited the growth at 10 μg while distilled water without extracts was used as a negative control and thus normal growth was recorded.
Table 5.
The MIC of various solvent extracts (mg/mL) of the bark of S. guineense
| Solvents | S. aureus | S. typhi | K. pneumoniae | E. coli |
|---|---|---|---|---|
| Acetone | 1.56 | 3.12 | 6.25 | 50 |
| Ethyl acetate | 12.5 | 12.5 | 12.5 | 50 |
| Chloroform | - | - | - | - |
| Ethanol | 3.12 | 6.25 | 1.56 | 50 |
| water | 6.25 | 3.12 | 6.25 | 50 |
(-) = No inhibition at 100 mg/mL
Fig. 4.
The MIC of the solvent extracts (mg/mL) of the bark of S. guineense against four different bacterial strains
Discussion
In this study, the dominant biologically useful phytochemicals and antibacterial activities of the bark of S. guineense, a traditionally useful plant in most rural parts of Ethiopia were quantitatively evaluated. It is well established that the information derived from various TM systems all over the world is utilized in the process of discovering lifesaving drugs [24]. The TM practiced today is maintained based on perceived safety profiles by the indigenous people which is based on its version of clinical trials, where the TMs have been used only when they have shown to be effective and safe [25]. It is agreeable that the practice of trial may take place for long periods and this might have helped mankind to retain a very detailed knowledge and understanding of many natural medicinal plants. The TM in use, however, needs rigorous validation studies for safe practice and wider application.
The fact that S. guineense is widely used in the TM all over the world [26, 27] is a remarkable indication that the plant has biologically active secondary metabolites as also revealed by the present study. Different parts of the plant reported in TM use include leaves [10, 28], roots [8], fruits [29], and the bark [11]. Among the diseases traditionally treated from preparations of different parts of S. guineense include diabetes [30], chronic diarrhea [31], malaria [10, 32], chest pain, stomachache and ringworm [33], hookworms [34], wounds [26], opportunistic infections [35], infertility, paresthesia and cancer [36].
The extract of the bark of the plant had high TPC and TFC especially the extract with more polar organic solvents. This is in agreement with previous studies in that different parts of the plant extracted with more polar solvents showed high TPC and TFC [26]. The TPC and TFC reported in the present study were found to be high when compared to the reports elsewhere [37]. The antioxidant properties are correlated with the phenolic content of any plants which act as hydrogen donors, and reducing agents, and are capable of scavenging free radicals [38, 39]. The TAC, increasing in a concentration-dependent manner, high DPPH activities, and FRAP are high in more polar organic solvent extracts in the present study. This finding is corroborated by previous studies [40–42].
Free radicals are generated in our body by various endogenous systems and a balance between free radicals and antioxidants is necessary for a proper physiological state. Recently, interest in flavonoids and other polyphenolic compounds has increased due to their potent antioxidant and free-radical scavenging activities [43, 44]. The high contents of TPC and TFC, and the TAC reported here make the S. guineense the potential source of antioxidants.
In the present study, the remarkable DPPH scavenging activity with the lowest EC50 recorded value of 5.62 μg/mL was very close to the positive control, ascorbic acid (5.27 μg/mL). This result further signifies that the plant can be a good source of free radical scavenging compounds and is supported by earlier reports [11]. The organo-protective effect of the bark extract reported elsewhere is also attributed to the high antioxidant activities of the plant and thus agrees with the present finding [45].
The results of the present research should be interpreted cautiously in that high doses of S. guineense extracts of different parts were reported with adverse indications elsewhere. For instance, the administration of high doses of the hydroethanolic extract of its leaves to the pregnant dams showed teratogenic effects and toxicity to the reproductive system in female rats [46, 47]. However, the effective doses in our finding are much less than the suggested high dose that resulted in adverse effects.
The present finding revealed that both Gram-positive (e.g., S. aureus), as well as Gram-negative bacteria strains (e.g., K. pneumonia) were found to be sensitive against the bark extracts of S. guineense with more polar solvents such as water, ethanol and acetone. Both groups of bacteria were found to be non-sensitive to less polar solvent extract (e.g., chloroform) which might indicate the biologically active principle is soluble in more polar solvents [48]. From very nature, Gram-negative bacteria have structural organizations that make them more resistant than Gram-positive bacteria making them the most important public health threat resulting in high morbidity and mortality worldwide. Therefore, it can be argued that the plant can be a potential source of lead materials for new drugs in a quest to fight drug resistance, an ever-present enormous global health crisis [49].
Conclusion
The present results indicate that the ethanol extract of the bark of S. guineense possesses high TPC and TFC endowing it with high antioxidant activities exhibited through DPPH and FRAP assays. The total antioxidant content was also high. The polar solvent extracts of the bark have shown antibacterial activities against both Gram-positive and Gram-negative bacterial species. Therefore, carefully designed further investigations, targeting a lead compound isolation and antioxidant compound characterization are recommended.
Abbreviations
- AAE
Ascorbic acid equivalents
- BHT
Butylated hydroxytoluene
- BHTE
Butylated hydroxytoluene equivalents
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- FRAP
Ferric-reducing antioxidant power
- MHA
Mueller Hinton Agar
- MIC
Minimum inhibitory concentration
- TAC
Total antioxidant capacity
- TFC
Total flavonoid content
- TM
Traditional medicine
Author contributions
Conceptual framework: ED, MM, HG, NT. Plant material collection, and extraction: MM, NT. Methodology: ED, MM, HG, NT. Phytochemistry experiments, spectrophotometry, and formal analysis: ED, MM, HG. Data curation – NT. Writing – original draft: NT. Writing – review & editing: ED, MM, HG, NT. All authors read and approved the final manuscript.
Funding
The authors declare that some costs incurred during this research were covered by the modest financial support obtained from the Hawassa College of Teacher Education (HCTE).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the research ethics committee of the Hawassa College of Teacher Education (Ref. №: HCTE/RIC/021/21). In addition, permission was obtained from local authorities to collect the plant material for this study. All the reagents used in this study were prepared, used, and disposed of according to the set laboratory guidelines and the material safety and data sheets (MSDS).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tabuti JR, Hassen IE, Pateh UU, Mahomoodally MF. Recent advances towards validating efficacy and safety of African traditional medicines. Evid Based Complement Altern Med. 2014;260567. 10.1155/2014/260567 [DOI] [PMC free article] [PubMed]
- 2.Giday M, Asfaw Z, Woldu Z. Ethnomedicinal study of plants used by Sheko ethnic group of Ethiopia. J Ethnopharmacol. 2010;132(1):75–85. 10.1016/j.jep.2010.07.046 [DOI] [PubMed] [Google Scholar]
- 3.Tuasha N, Petros B, Asfaw Z. Medicinal plants used by traditional healers to treat malignancies and other human ailments in Dalle District, Sidama Zone. Ethiopia J Ethnobiol Ethnomed. 2018;14(15). 10.1186/s13002-018-0213-z [DOI] [PMC free article] [PubMed]
- 4.WHO. WHO traditional medicine strategy: 2014–2023. Geneva, Switzerland: World Health Organization;: World Health Organization; 2013. [Google Scholar]
- 5.Abebe D. Traditional medicine in Ethiopia: the attempts being made to promote it for effective and better utilization. Sinet. 1986;9(Suppl):61–9. [Google Scholar]
- 6.Tuasha N, Fekadu S, Deyno S. Prevalence of herbal and traditional medicine in Ethiopia: a systematic review and meta-analysis of 20-year studies. Syst Rev. 2023;12:232. 10.1186/s13643-023-02398-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bishaw M. Promoting traditional medicine in Ethiopia: a brief historical review of government policy. Soc Sci Med. 1991;33(2):193–200. 10.1016/0277-9536(91)90180-k [DOI] [PubMed] [Google Scholar]
- 8.Abera B, Adane L, Mamo F. Phytochemical investigation the root extract of Syzygium guineense and isolation of 2, 3, 23-trihydroxy methyl oleanate. J Pharmacogn Phytochem. 2018;7(2):3104–11. [Google Scholar]
- 9.Oladosu I, Lawson L, Aiyelaagbe O, Emenyonu N, Afieroho O. Anti-tuberculosis lupane-type isoprenoids from Syzygium Guineense Wild DC.(Myrtaceae) stem bark. Future J Pharm Sci. 2017;3(2):148–52. 10.1016/j.fjps.2017.05.002 [Google Scholar]
- 10.Tadesse SA, Wubneh ZB. Antimalarial activity of Syzygium guineense during early and established Plasmodium infection in rodent models. BMC Complement Altern Med. 2017;17:1–7. 10.1186/s12906-016-1538-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pieme CA, Ngoupayo J, Khou-Kouz Nkoulou CH, Moukette Moukette B, Njinkio Nono BL, Ama Moor VJ, Ze Minkande J, Yonkeu Ngogang J. Syzyguim guineense extracts show antioxidant activities and beneficial activities on oxidative stress induced by ferric chloride in the liver homogenate. Antioxidants. 2014;3(3):618–35. 10.3390/antiox3030618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ezenyi IC, Mbamalu ON, Balogun L, Omorogbe L, Ameh F, Salawu O. Antidiabetic potentials of Syzygium guineense methanol leaf extract. J Phytopharmacol. 2016;5(4):150–6. 10.31254/phyto.2016.5406 [Google Scholar]
- 13.Tsakala T, Penge O, John K. Screening of in vitro antibacterial activity from Syzygium Guineense (Willd) hydrosoluble dry extract. Ann Pharm Fr. 1996;54(6):276-9. PMID: 9008903. [PubMed]
- 14.Maregesi S, Kagashe G, Messo CW, Mugaya L. Determination of mineral content, cytotoxicity and anthelmintic activity of Syzygium guineense fruits. Saudi J Med Pharm Sci. 2016;2(5):95–9. 10.36348/sjmps.2016.v02i05.001 [Google Scholar]
- 15.IOR I, Otimenyin I, Umar M. Anti-inflammatory and analgesic activities of the ethanolic extract of the leaf of Syzygium guineense in rats and mice. IOSR J Pharm. 2012;2(2):33–6. [Google Scholar]
- 16.Aung EE, Kristanti AN, Aminah NS, Takaya Y, Ramadhan R. Plant description, phytochemical constituents and bioactivities of Syzygium genus: a review. Open Chem. 2020;18(1):1256–81. 10.1515/chem-2020-0175 [Google Scholar]
- 17.Edwards S, Tadesse M, Hedberg I. Flora of Ethiopia and Eritrea, volume 2, part 2: Canellaceae to Euphorbiaceae. Uppsala: The National Herbarium, Addis Ababa, Ethiopia, and Department of Systematic Botany; 1995. [Google Scholar]
- 18.Engeda D, Geremew B, Gulelat D, Rupasinghe V. Antioxidant and α-amylase inhibition activities in vitro of various solvent extracts of Thymus schimperi Ronniger. J Med Plants Res. 2015;9(15):515–24. 10.5897/JMPR2014.5431 [Google Scholar]
- 19.Ayoola G, Coker H, Adesegun S, Adepoju-Bello A, Obaweya K, Ezennia EC, Atangbayila T. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop J Pharm Res. 2008;7(3):1019–24. 10.4314/tjpr.v7i3.14686 [Google Scholar]
- 20.Beyazen A, Dessalegn E, Mamo W. Phytochemical screening and biological activities of leaf of Foeniculum vulgare (Ensilal). World J Agri Sci. 2017;13(1):01–10. 10.5829/idosi.wjas.2017.01.10 [Google Scholar]
- 21.FR WC. Performance standards for antimicrobial susceptibility testing; twenty-second informational supplement. Clin Lab Stand Inst (CLSI). 2012;32:M100. [Google Scholar]
- 22.Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48(1):5–16. 10.1093/jac/48.suppl_1.5 [DOI] [PubMed] [Google Scholar]
- 23.Gupta N, Shrivastava N, Singh PK, Bhagyawant SS. Phytochemical evaluation of moth bean (Vigna aconitifolia L.) seeds and their divergence. Biochem Res Int. 2016;3136043. 10.1155/2016/3136043 [DOI] [PMC free article] [PubMed]
- 24.Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001;109(1):69–75. 10.1289/ehp.01109s169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chivian E. The Role of Traditional Medicine in Drug Discovery https://blog.oup.com/2008/11/drug_discovery/ Oxford University Press (2008). UK, England. -Accessed: July 07, 2023.
- 26.Nguyen TL, Rusten A, Bugge MS, Malterud KE, Diallo D, Paulsen BS, Wangensteen H. Flavonoids, gallotannins and ellagitannins in Syzygium guineense and the traditional use among Malian healers. J Ethnopharmacol. 2016;192:450–58. 10.1016/j.jep.2016.09.035 [DOI] [PubMed] [Google Scholar]
- 27.Uddin AN, Hossain F, Reza AA, Nasrin MS, Alam AK. Traditional uses, pharmacological activities, and phytochemical constituents of the genus Syzygium: a review. Food Sci Nutr. 2022;10(6):1789–819. 10.1002/fsn3.2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loha M, Mulu A, Abay SM, Ergete W, Geleta B. Acute and subacute toxicity of methanol extract of Syzygium guineense leaves on the histology of the liver and kidney and biochemical compositions of blood in rats. Evid Based Complement Altern Med. 2019;5702159. 10.1155/2019/5702159 [DOI] [PMC free article] [PubMed]
- 29.Ruffo CK, Birnie A, Tengnäs B. Edible wild plants of Tanzania. Reg Land Manage Unit/Sida. 2002;Issue 27 of RELMA technical handbook series.
- 30.Gbolade AA. Inventory of antidiabetic plants in selected districts of Lagos State, Nigeria. J Ethnopharmacol. 2009;121(1):135–39. 10.1016/j.jep.2008.10.013 [DOI] [PubMed] [Google Scholar]
- 31.Ezenyi IC, Igoli JO. Antidiarrhoeal properties of Syzygium guineense leaf extract and identification of chemical constituents in its active column fractions. J Complement Integr Med. 2018;16(2):20160074. 10.1515/jcim-2016-0074 [DOI] [PubMed] [Google Scholar]
- 32.Kasali FM, Mahano AO, Nyakabwa DS, Kadima NJ, Misakabu FM, Tshibangu DST, Ngbolua KN, Mpiana PT. Ethnopharmacological survey of medicinal plants used against malaria in Bukavu City (DR Congo). Eur J Med Plants. 2014;4(1):29–44. [Google Scholar]
- 33.Hamill F, Apio S, Mubiru N, Mosango M, Bukenya-Ziraba R, Maganyi O, Soejarto D. Traditional herbal drugs of southern Uganda, I. J Ethnopharmacol. 2000;70(3):281–300. 10.1016/s0378-8741(00)00180-x [DOI] [PubMed] [Google Scholar]
- 34.Mukherjee PK, Saha K, Murugesan T, Mandal S, Pal M, Saha B. Screening of anti-diarrhoeal profile of some plant extracts of a specific region of West Bengal, India. J Ethnopharmacol. 1998;60(1):85–9. 10.1016/s0378-8741(97)00130-x [DOI] [PubMed] [Google Scholar]
- 35.Chinsembu KC, Hedimbi M. An ethnobotanical survey of plants used to manage HIV/AIDS opportunistic infections in Katima Mulilo, Caprivi region, Namibia. J Ethnobiol Ethnomed. 2010;6(1):1–9. 10.1186/1746-4269-6-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kigen G, Kipkore W, Wanjohi B, Haruki B, Kemboi J. Medicinal plants used by traditional healers in Sangurur, Elgeyo Marakwet County, Kenya. Pharmacognosy Res. 2017;9(4):333–47. 10.4103/pr.pr_42_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edewor T, Akintola A, Ogundola A, Ibikunle G, Adepoju A, Mmuo A, Owa S. Phytochemical constituents, total flavonoid and phenolic contents and antioxidant activity of leaves of Syzygium guineense. J Pharmacogn Phytochem. 2021;10(4):127–32. [Google Scholar]
- 38.Wojdyło A, Oszmiański J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105(3):940–49. 10.1016/j.foodchem.2007.04.038 [Google Scholar]
- 39.Phuyal N, Jha PK, Raturi PP, Rajbhandary S. Total phenolic, flavonoid contents, and antioxidant activities of fruit, seed, and bark extracts of Zanthoxylum armatum DC. Sci World J. 2020;8780704. 10.1155/2020/8780704 [DOI] [PMC free article] [PubMed]
- 40.Wakeel A, Jan SA, Ullah I, Shinwari ZK, Xu M. Solvent polarity mediates phytochemical yield and antioxidant capacity of Isatis tinctoria. PeerJ. 2019;7:e7857. 10.7717/peerj.7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nawaz H, Shad MA, Rehman N, Andaleeb H, Ullah N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Braz J Pharm Sci. 2020;56:e17129. 10.1590/s2175-97902019000417129 [Google Scholar]
- 42.Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, Ju Y-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal. 2014;22(3):296–302. 10.1016/j.jfda.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4(8):118–26. 10.4103/0973-7847.70902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alkadi H. A review on free radicals and antioxidants. Infect Disord Drug Targets. 2020;20(1):16–26. 10.2174/1871526518666180628124323 [DOI] [PubMed] [Google Scholar]
- 45.Tankeu FN, Pieme CA, Biapa Nya CP, Njimou RJ, Moukette BM, Chianese A, Ngogang JY. In vitro organo-protective effect of bark extracts from Syzygium guineense var macrocarpum against ferric-nitrilotriacetate-induced stress in Wistar rats homogenates. BMC Complement Altern Med. 2016;16:315. 10.1186/s12906-016-1263-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abebe M, Asres K, Bekuretsion Y, Woldkidan S, Debebe E, Seyoum G. Teratogenic effect of high dose of Syzygium guineense (myrtaceae) leaves on wistar albino rat embryos and fetuses. Evid Based Complement Altern Med. 2021;6677395. 10.1155/2021/6677395 [DOI] [PMC free article] [PubMed]
- 47.Abebe MS, Asres K, Bekuretsion Y, Woldekidan S, Debebe E, Abebe A, Sisay B, Seyoum G. Toxic effect of Syzygium guineense ethanolic extract on female reproduction in rats: an evidence from a 10 week repeated-dose toxicity study. Heliyon 9(6):e17335. 10.1016/j.heliyon.2023.e17335 [DOI] [PMC free article] [PubMed]
- 48.Borges A, José H, Homem V, Simões M. Comparison of techniques and solvents on the antimicrobial and antioxidant potential of extracts from Acacia dealbata and Olea europaea. Antibiotics (Basel). 2020;9(2):48. 10.3390/antibiotics9020048 [DOI] [PMC free article] [PubMed]
- 49.Breijyeh Z, Jubeh B, Karaman R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules. 2020;25(6):1340. 10.3390/molecules25061340 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.