Summary
Multiple sclerosis (MS) is an autoimmune inflammatory demyelinating disease that results in motor, sensory, cognitive, and affective deficits. Hippocampal demyelination, a common occurrence in MS, is linked to impaired cognitive function and mood. Despite this, the precise mechanisms underlying cognitive impairments in MS remain elusive. Pleiotrophin (PTN), secreted by neural stem cells and astrocytes, plays a crucial role in regulating cognition. This study investigates the role of astrocyte-derived PTN. We found that genetic deletion of astrocyte-derived PTN hinders hippocampal neurogenesis. Additionally, conditional ablation of PTN in astrocytes exacerbates neurogenic deficits in the demyelinated hippocampus. Importantly, overexpression of PTN in astrocytes reverses neurogenic and cognitive impairments caused by demyelination, underscoring PTN’s protective role in MS. PTN cooperates with protein tyrosine phosphatase receptor type Z1 (PTPRZ1) or anaplastic lymphoma kinase (ALK) receptors to activate the AKT signaling pathway, thereby enhancing hippocampal neurogenesis and cognition in demyelinated mice. These findings illuminate novel effects of astrocyte-derived PTN on hippocampal neurogenesis and cognition.
Keywords: pleiotrophin, multiple sclerosis, demyelination, hippocampal neurogenesis, cognition
Graphical abstract

Highlights
-
•
Astrocytic Ptn knockdown impairs hippocampal neurogenesis
-
•
Astrocytic Ptn deletion exacerbates neurogenic deficits in MS model
-
•
PTN rescues neurogenic and cognitive deficits in the demyelinated hippocampus
-
•
PTN interacts with PTPRZ1/ALK to enhance neurogenesis by activating AKT signaling
Tang et al. demonstrate that astrocyte-derived PTN engages with the PTPRZ1/ALK receptor to ameliorate neurogenesis and cognition by activating AKT signaling in the demyelinated hippocampus.
Introduction
Multiple sclerosis (MS) is a chronic immune-mediated disease of the central nervous system (CNS) characterized by demyelination, neuronal and axonal injury, and astroglial activation (Jakimovski et al., 2024; Linnerbauer et al., 2020; Qian et al., 2023). During MS pathogenesis, activated astrocytes not only enhance microglial clearance of myelin debris but also regulate synapse number and activity (Skripuletz et al., 2013; Williams and Featherstone, 2014). Notably, hippocampal demyelination occurs in over half of patients with MS and is linked to cognitive decline (Di Filippo et al., 2018; Dutta et al., 2011; Papadopoulos et al., 2009). Cognitive impairments, affecting 30%–70% of patients with MS, include deficits in memory, information processing speed, attention, learning, and increased anxiety and depressive behaviors (Benedict et al., 2020; Jakimovski et al., 2024; Planche et al., 2016). These deficits significantly reduce the quality of life and impose a substantial economic burden on patients. Despite long-standing recognition of cognitive damage in MS, the precise mechanisms underlying these impairments remain unclear.
Adult hippocampal neurogenesis (AHN), a unique CNS process, involves the proliferation and differentiation of neural stem cells (NSCs) and the development and integration of newborn neurons (Ming and Song, 2011; Rocca et al., 2018). AHN is crucial for maintaining CNS homeostasis and synaptic plasticity, and it is associated with cognitive and affective processes (Anacker and Hen, 2017; Ming and Song, 2011; Rocca et al., 2018). Remarkably, studies have noted functional disconnection of the hippocampus, neuronal loss, and decreased synaptic densities in MS and cuprizone (CPZ) demyelinated mice, suggesting that hippocampal demyelination may disrupt neurogenesis. In line with this, our study focuses on alterations in hippocampal neurogenesis and functional connectivity in CPZ mice, where demyelination inhibited hippocampal neurogenesis, newborn neuron development, and synaptic connectivity.
Pleiotrophin (PTN), a neurotrophic cytokine, is known for its protective role in various diseases (Pufe et al., 2003; Reyes-Mata et al., 2022; Skillback et al., 2017; Wang et al., 2020; Zhang et al., 2024). Increasing evidence shows PTN upregulation in nervous system diseases, particularly MS and its animal models, such as experimental autoimmune encephalomyelitis (EAE) and CPZ models (Baltan et al., 2021; Linnerbauer et al., 2021; Liu et al., 1998; Wheeler et al., 2020). PTN regulates multiple CNS functions (Li et al., 2023; Qin et al., 2017; Tang et al., 2019). Our previous studies identified PTN’s high expression in NSCs, where it promotes NSC proliferation and differentiation, newborn neuron development, and hippocampal neuron connectivity, ultimately enhancing cognitive functions (Li et al., 2023; Tang et al., 2019). Interestingly, PTN is also highly expressed in astrocytes, besides NSCs, in both MS and its EAE and CPZ models (Baltan et al., 2021; Linnerbauer et al., 2021; Wheeler et al., 2020). However, the role of astrocyte-secreted PTN in regulating hippocampal neurogenesis and cognitive deficits in MS remains elusive.
This study aims to elucidate the role of astrocyte-derived PTN in the demyelinated hippocampus. We observed elevated PTN levels in the demyelinated hippocampus, along with impaired hippocampal neurogenesis and newborn neuron development. Conditional ablation of PTN in astrocytes inhibited hippocampal neurogenesis and contributed to cognitive decline. Deletion of PTN from astrocytes exacerbated hippocampal neurogenesis deficits and cognitive impairments induced by hippocampal demyelination. Conversely, PTN overexpression reversed these negative effects in the demyelinated model. Consistent with previous findings (Li et al., 2023; Tang et al., 2019), PTN promoted NSC proliferation and differentiation, and newborn neuron development via AKT signaling activation by binding to protein tyrosine phosphatase receptor type Z1 (PTPRZ1) and anaplastic lymphoma kinase (ALK) receptors. Furthermore, AKT signaling activation rescued abnormal hippocampal neurogenesis and cognitive function deficits.
Results
PTN is elevated in MS and is correlated with neurocognitive assessments
Numerous studies have identified elevated expression levels of PTN in MS, Alzheimer’s disease (AD), breast cancer, osteoarthritis, systemic lupus erythematosus, and other diseases (Pufe et al., 2003; Reyes-Mata et al., 2022; Skillback et al., 2017; Wang et al., 2020; Zhang et al., 2024). To evaluate the role of PTN in MS, we enrolled 78 patients with MS and 55 healthy controls (HCs) in this study. Their baseline and clinical characteristics are detailed in Table S1. There were no significant differences in age, gender, and years of education between the two groups (Table S1). Notably, PTN levels in serum and cerebrospinal fluid (CSF), evaluated by ELISA, were significantly elevated in patients with MS compared to HCs (Figure 1A; Table S1), consistent with previous findings (Reyes-Mata et al., 2022). Additionally, we re-analyzed PTN mRNA expression using single-cell RNA sequencing of astrocytes from patients with MS and HCs (Wheeler et al., 2020). This analysis also revealed upregulated PTN expression in MS compared to HCs (Figure 1G).
Figure 1.
PTN levels in MS and the demyelinated hippocampus
(A) Serum and CSF PTN expression levels in patients with MS and healthy controls (HCs) measured by ELISA. Sample sizes: HC-serum, N = 28; MS-serum, N = 56; HC-CSF, N = 25; MS-CSF, N = 29.
(B–F) Correlation between serum PTN levels and neuropsychological assessments in patients with MS.
(G) Human astrocyte RNA-seq analysis showing elevated PTN expression in patients with MS.
(H and I) Schematic and timeline for assaying hippocampal neurogenesis and dendritic development in 6-week-old wild-type mice on a chow or CPZ diet with rapamycin (Rap).
(J) Representative images and quantification of MBP expression in the hippocampus after CPZ and Rap treatment. n = 5 per group. Scale bar, 100 μm.
(K) PTN levels measured by ELISA in acutely dissected, unfixed hippocampal tissues from mouse brains. n = 5 per group.
(L) Representative images and quantification of PTN expression in the hippocampus following CPZ and Rap treatment. n = 5 per group. Scale bar, 50 μm.
(M) Western blot analysis of MBP and PTN expression in hippocampal lysates from Chow/Rap and CPZ/Rap mice. n = 4 experiments per group.
(N and O) Schematic and timeline for assaying hippocampal neurogenesis and dendritic development in 6-week-old wild-type mice injected with vehicle or LPC.
(P) Representative images and quantification of MBP expression in the hippocampus post-control or LPC injection. n = 5 per group. Scale bar, 50 μm.
(Q) Representative images and quantification of PTN expression in the hippocampus post LPC injection. n = 5 per group. Scale bar, 50 μm.
(R) Quantification of MBP and PTN expression by immunoblotting in hippocampal lysates from control or LPC mice. n = 4 experiments per group. Data in (A), (J), (K), (L), (M), (P), (Q), and (R) are presented as mean ± SEM. Statistical significance was evaluated with Student’s t test for two-group comparisons. Non-significant comparisons are not indicated. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
PTN has been reported to regulate cognitive functions in AD (Krellman et al., 2014; Li et al., 2023). To explore the correlation between PTN levels and cognition in MS, participants underwent cognitive assessments including the Mini-Mental State Examination (MMSE), Symbol Digit Modalities Test (SDMT), and Brief Visuospatial Memory Test-Revised (BVMT-R). Emotional performance was measured using the Hamilton Anxiety Rating Scale (HARS) and the Hamilton Depression Rating Scale (HDRS). Interestingly, serum PTN levels were negatively correlated with anxiety and depressive scores (HARS and HDRS) and positively correlated with learning and memory scores (MMSE, SDMT, and BVMT-R) (Figures 1B–1F). In summary, PTN may exert neuroprotective functions and play a role in regulating cognitive and affective deficits in MS.
PTN levels and hippocampal neurogenesis changes in the demyelinated model in vivo
To explore PTN expression in a demyelinated model, we established a CPZ animal model in wild-type C57BL/6J mice by feeding them a CPZ diet for 6 weeks, followed by rapamycin (Rap) injection (Figures 1H and 1I). Additionally, we used a second demyelinated model by stereotaxic injection of the myelin toxin lysolecithin (LPC) into the hippocampus (Figures 1N and 1O). Consistent with previous results, both CPZ/Rap and LPC models exhibited decreased levels of myelin basic protein (MBP), indicating significant myelin loss in the hippocampal dentate gyrus (DG) (Figures 1J and 1P). This was corroborated by downregulated MBP expression assessed through western blot analysis of hippocampal lysates from both models (Figures 1M and 1R). Previous studies have shown elevated PTN in both the CPZ model and EAE (Baltan et al., 2021; Linnerbauer et al., 2021; Liu et al., 1998). Similarly, PTN levels measured by ELISA were upregulated in the hippocampus of the CPZ/Rap group (Figure 1K), consistent with western blot results (Figures 1M and 1R). Furthermore, PTN was highly expressed in GFAP+ astrocytes in the demyelinated models, as evidenced by immunostaining (Figures 1L and 1Q).
We then focused on neurogenesis in the demyelinated hippocampus. CPZ/Rap or LPC administration impeded NSC proliferation in mice labeled with 5-ethynyl-2′-deoxyuridine (EdU), resulting in fewer EdU+ cells, EdU+GFAP+Sox2+ radial glia-like (RGL) NSCs, and transiently amplifying progenitor (TAP) EdU+GFAP−Sox2+ cells in the DG (Figures S1A and S1F). For NSC differentiation analysis, bromodeoxyuridine (BrdU) was injected into control and demyelinated mice. This revealed significantly fewer BrdU+DCX+ immature neurons and BrdU+NeuN+ mature neurons in demyelinated mice (Figures S1B, S1C, S1G, and S1H), indicating inhibited NSC differentiation.
Further characterization of newborn hippocampal neurons, labeled with red fluorescent protein (RFP)-expressing retroviruses, during demyelination showed reduced neuronal dendritic length and complexity in CPZ/Rap or LPC-treated mice compared to controls (Figures S1D and S1I). Additionally, synaptic connectivity analysis revealed significantly reduced dendritic spine density in CPZ/Rap and LPC-induced mice compared to controls (Figures S1E and S1J). Therefore, demyelination induced by CPZ/Rap or LPC impedes hippocampal neurogenesis, inhibiting NSC proliferation and differentiation and damaging the development of newborn hippocampal neurons.
Deletion of PTN from astrocytes impedes hippocampal neurogenesis and development of newborn neurons
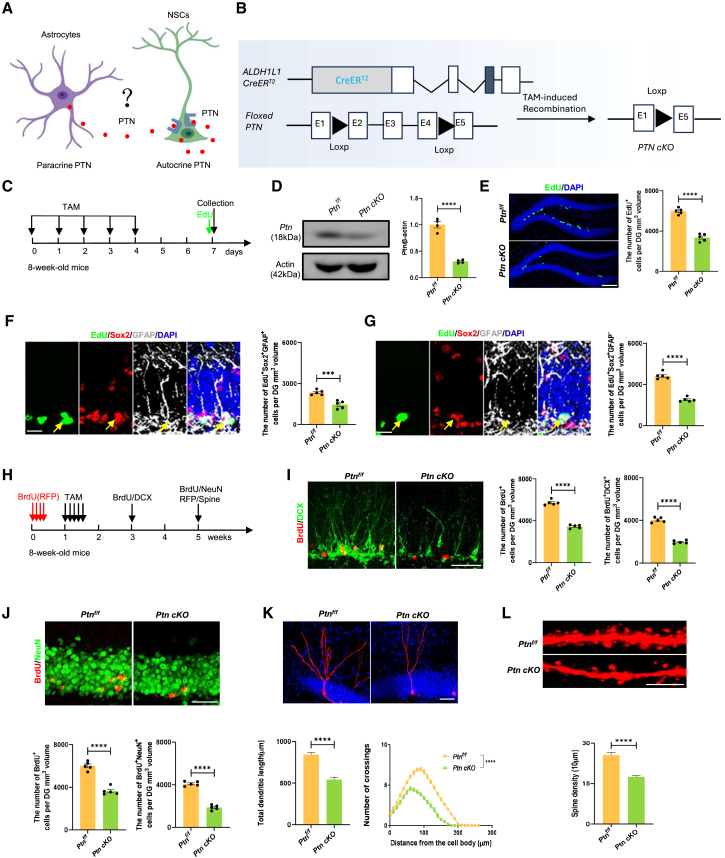
Previous studies demonstrated that PTN is highly expressed in NSCs and promotes hippocampal neurogenesis in AD (Li et al., 2023; Tang et al., 2019). Notably, PTN is also highly expressed in astrocytes (Figure 2A). ALDH1L1-CreERT2 transgenic mice have been proved to be valuable tools to selectively target astrocytes with no detectable NSC contamination (Beyer et al., 2021; Srinivasan et al., 2016). To elucidate the role of astrocyte-secreted PTN in hippocampal neurogenesis, we used astrocyte-specific ALDH1L1-CreERT2 transgenic mice to remove PTN from astrocytes. We generated conditional knockout (cKO) mice by crossing Ptnf/f mice with ALDH1L1-CreERT2 mice, inducing PTN deletion in astrocytes upon tamoxifen administration (Figure 2B). As expected, PTN levels were significantly reduced in Ptn cKO mice, as confirmed by western blot, demonstrating efficient knockout (Figure 2D). Notably, PTN in astrocytes, rather than in NSCs, was specifically knocked out in Ptn cKO mice, regardless of Chow/Rap or CPZ/Rap treatment (Figures S2A and S2B). We first evaluated the effects of astrocyte-specific PTN deletion on NSC proliferation. There was a notable decrease in the number of EdU+ cells, EdU+GFAP+Sox2+ RGL NSCs, and EdU+GFAP−Sox2+ TAP cells in Ptn cKO mice compared to Ptnf/f controls (Figures 2C and 2E–2G). To explore PTN’s effects on neuronal differentiation, we administered BrdU before tamoxifen injection and carried out immunofluorescence analysis (Figure S2H). As expected, the number of BrdU+DCX+ immature neurons and BrdU+NeuN+ mature neurons was significantly reduced in Ptn cKO mice compared to controls (Figures 2I and 2J).
Figure 2.
Effects of astrocytic Ptn deletion on adult hippocampal neurogenesis and newborn neuron development
(A) Diagram showing PTN’s role as secreted by NSCs (autocrine) and astrocytes (paracrine) on NSCs.
(B) Schematic of conditional Ptn knockout in astrocytes. Ptnf/f mice were crossed with ALDH1L1-CreERT2 mice to generate astrocytic Ptn deletion (Ptn cKO) after tamoxifen injection.
(C) Timeline for assaying NSC proliferation in the DG of Ptnf/f and Ptn cKO mice post tamoxifen injection.
(D) Validation of PTN knockout in hippocampal tissues via immunoblotting, showing PTN levels relative to β-actin in Ptn cKO and Ptnf/f mice. n = 4 independent experiments.
(E) Images and quantification of EdU+ cells in the DG of Ptnf/f and Ptn cKO mice 2 h post EdU injection. n = 5 animals per group. Scale bars, 100 μm.
(F) Images and quantification of EdU+GFAP+Sox2+ RGL cells in the DG of Ptn cKO and Ptnf/f mice. n = 5 animals per group. Scale bar, 20 μm.
(G) Images and quantification of EdU+GFAP−Sox2+ TAP cells in the DG of Ptn cKO and Ptnf/f mice. n = 5 animals per group. Scale bar, 20 μm.
(H) Timeline for BrdU/RFP labeling and tamoxifen injection to assess NSC differentiation.
(I and J) Images and quantification of BrdU+ cells, BrdU+DCX+ immature neurons, and BrdU+NeuN+ mature newborn neurons in the DG of Ptn cKO and Ptnf/f mice. n = 5 animals per group. Scale bar, 50 μm.
(K) Images and quantification of dendritic length and complexity of RFP+ newborn neurons in Ptn cKO and Ptnf/f mice (two-way ANOVA, p < 0.0001). n = 30 neurons per group from 5 mice. Scale bars, 50 μm.
(L) Images and quantification of dendritic spine density of newborn neurons in Ptn cKO and Ptnf/f mice. n = 30 neurons per group from 5 mice. Scale bars, 5 μm. Data in (D–G) and (I–L) are presented as mean ± SEM; statistical significance was calculated using Student’s t test for two-group comparisons; for (K), dendritic complexity was evaluated with two-way ANOVA. Non-significant comparisons are not indicated. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
We further investigated the effect of astrocyte-derived PTN on the development of newborn neurons. PTN deficiency dramatically impaired neuronal dendritic length, complexity, and spine density (Figure 2K and 2L). These results suggest that PTN is crucial for hippocampal neurogenesis and the development of newborn neurons.
Deletion of astrocyte-derived PTN aggravates neurogenic deficits in the demyelinated hippocampus
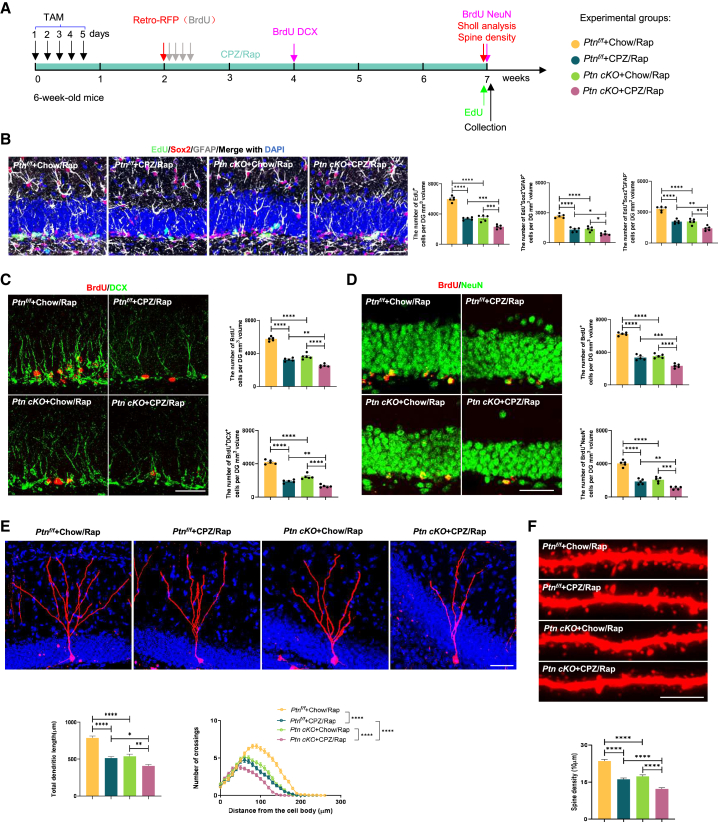
To assess the effect of PTN on the demyelinated animal model, we used astrocyte Ptn cKO mice and induced CPZ/Rap and LPC demyelinated models in these mice (Figures 3A and S2C). Notably, deletion of PTN from astrocytes significantly aggravated neurogenic defects during demyelination. This was evidenced by a reduced number of EdU+ cells, RGL NSCs, and TAP cells compared to Ptnf/f controls with or without CPZ/Rap or LPC administration (Figures 3B and S2D).
Figure 3.
Effects of astrocytic Ptn deletion on adult hippocampal neurogenesis and newborn neuron development in the CPZ/Rap mouse model
(A) Timeline of experiments assessing adult hippocampal neurogenesis and newborn neuron development in Ptn cKO mice following CPZ/Rap treatment.
(B) Images and quantification of EdU+ cells, EdU+GFAP+Sox2+ RGL cells, and EdU+GFAP−Sox2+ TAP cells in the DG of four groups. n = 5 animals per group. Scale bar, 50 μm.
(C) Images and quantification of BrdU+ cells and BrdU+DCX+ immature neurons in the DG of indicated groups. n = 5 animals per group. Scale bar, 50 μm.
(D) Images and quantification of BrdU+ cells and BrdU+NeuN+ mature newborn neurons in the DG of indicated groups. n = 5 animals per group. Scale bar, 50 μm.
(E) Images and quantification of dendritic length and complexity of RFP+ newborn neurons (two-way ANOVA: Ptnf/f + Chow/Rap vs. Ptnf/f + CPZ/Rap, p < 0.0001; Ptnf/f + CPZ/Rap vs. Ptn cKO + CPZ/Rap, p < 0.0001; Ptn cKO + Chow/Rap vs. Ptn cKO + CPZ/Rap, p < 0.0001). n = 30 neurons per group from 5 mice. Scale bars, 50 μm.
(F) Images and quantification of dendritic spine density of newborn neurons. n = 30 neurons per group from 5 mice. Scale bars, 5 μm. Data in (B–F) are presented as mean ± SEM; statistical significance was computed with one-way ANOVA and Tukey’s post hoc multiple comparisons; for (E), dendritic complexity was evaluated with two-way ANOVA. Non-significant comparisons are not indicated. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
Furthermore, deletion of astrocyte-derived PTN exacerbated the inhibition of NSC differentiation induced by demyelination, resulting in a notable reduction of immature (BrdU+DCX+) and mature (BrdU+NeuN+) newborn neurons (Figures 3C, 3D, S2E, and S2F). Analysis of the dendritic morphology and spine density of hippocampal newborn neurons revealed that the deletion of astrocyte-secreted PTN further worsened the impairments in neuron development caused by demyelination (Figures 3E, 3F, S2G, and S2H).
Based on these findings, we conclude that the deletion of astrocyte-derived PTN significantly aggravates neurogenic deficits in the context of demyelination.
PTN overexpression ameliorates neurogenic and cognitive deficits in the demyelinated hippocampus
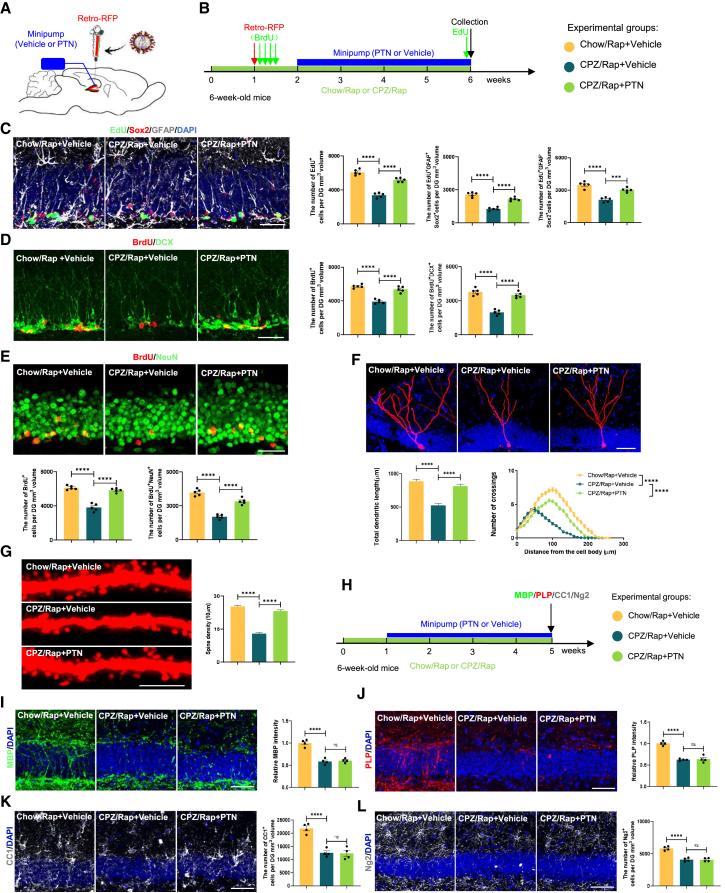
Considering the detrimental effects of PTN deletion on neurogenesis in the demyelinated hippocampus, we hypothesized that PTN exerts neuroprotective effects on hippocampal neurogenesis and the development of newborn neurons. To explore PTN’s therapeutic potential in a demyelinated animal model, we stereotaxically implanted a mini pump of PTN into the hippocampus of CPZ/Rap demyelinated mice for 4 weeks (Figures 4A and 4B). Importantly, we reconstructed an adeno-associated virus (AAV)-RNA vector to overexpress PTN under the control of the GfaABC1D promoter (astrocytic specific) (Figure S3A). To further evaluate the impact of astrocyte-derived PTN on neurogenesis, we stereotaxically injected a PTN overexpression AAV (PTN-AAV) specifically targeting astrocytes into the hippocampus of CPZ/Rap demyelinated mice (Figure S3B). As expected, PTN-AAV was specifically expressed in astrocytes rather than RGL NSCs in the DG of the hippocampus (Figure S3C), leading to PTN overexpression in astrocytes of the hippocampus through immunostaining of PTN (green) and astrocytic marker GFAP (gray) (Figure S3D). PTN overexpression in hippocampal tissues was validated via immunoblotting, with a satisfactory efficiency (Figure S3E). Our results revealed that both PTN-AAV and mini osmotic pump of PTN attenuated the negative effects of CPZ/Rap treatment. Specifically, PTN overexpression restored NSC proliferation (Figures 4C and S3F) and differentiation (Figures 4D, 4E, S3G, and S3H) and improved newborn neuron development (Figures 4F, 4G, S3I, and S3J).
Figure 4.
Effects of continuous transfusion of PTN via mini pump on hippocampal neurogenesis and newborn neuron development affected by CPZ/Rap administration
(A and B) Schematic and timeline of experiments to assay hippocampal neurogenesis and newborn neuron development in 6-week-old wild-type mice receiving mini-pump infusion of vehicle or PTN after CPZ/Rap treatment.
(C) Images and quantification of EdU+ cells, EdU+GFAP+Sox2+ RGL cells, and EdU+GFAP−Sox2+ TAP cells in the DG of three groups. n = 5 animals per group. Scale bar, 50 μm.
(D) Images and quantification of BrdU+ cells and BrdU+DCX+ immature neurons in the DG of three groups. n = 5 animals per group. Scale bars, 50 μm.
(E) Images and quantification of BrdU+ cells and BrdU+NeuN+ mature newborn neurons in the DG of indicated groups. n = 5 animals per group. Scale bars, 50 μm.
(F) Images and quantification of dendritic length and complexity of RFP+ newborn neurons in indicated groups (two-way ANOVA: Chow/Rap + Vehicle vs. CPZ/Rap diet + Vehicle, p < 0.0001; CPZ/Rap + Vehicle vs. CPZ/Rap diet + PTN, p < 0.0001). n = 30 neurons per group from 5 mice. Scale bars, 50 μm.
(G) Images and quantification of dendritic spine density of newborn neurons in indicated groups. n = 30 neurons per group from 5 mice. Scale bars, 5 μm.
(H) Timeline of experiments to assay myelination in 6-week-old wild-type mice receiving mini-pump infusion of vehicle or PTN after CPZ/Rap treatment.
(I–L) Images and quantification of myelin protein expression (MBP and PLP) and the number of oligodendrocytes (Ng2 and CC1) in the DG of mice receiving mini-pump infusion of vehicle or PTN after CPZ/Rap treatment. n = 4 animals per group. Scale bar, 50 μm. Data in (C–L) are presented as mean ± SEM; statistical significance was computed with one-way ANOVA and Tukey’s post hoc multiple comparisons; for (F), dendritic complexity was evaluated with two-way ANOVA. Non-significant comparisons are not indicated. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
To investigate PTN’s effect on myelination, we stereotaxically injected mini-pump infusion of PTN to DG of mice 1 week after CPZ/Rap administration (Figure 4H). We focused on myelination by assessing the density of myelin proteins labeled with MBP and proteolipid protein (PLP) and the number of oligodendrocytes. Ng2+ oligodendrocyte precursor cells (OPCs) are crucial for myelination as they proliferate and differentiate into CC1+ mature oligodendrocytes (Figure 4H). CPZ/Rap exposure resulted in significant reductions in MBP (Figures 4I and S4A), PLP (Figures 4J and S4B), CC1+ mature oligodendrocytes (Figures 4K and S4C), and Ng2+ OPCs (Figures 4L and S4D). However, PTN administration did not improve demyelination induced by CPZ/Rap treatment.
Additionally, previous studies have observed microglial activation and cell apoptosis in the hippocampus of CPZ/Rap demyelinated mice (Adebiyi and Bynoe, 2023; Zhang et al., 2020). We thereafter investigated the role of PTN in cell apoptosis via terminal deoxynucleotidyl transferase DUTP nick end labeling (TUNEL) staining to count the number of TUNEL-positive cells and microglial activation by performing immunofluorescence to quantify the number and size of Iba1+ microglia. The results demonstrated that activated microglial cells (increased number and size of Iba1+ microglia) and cell apoptosis rate in the hippocampus were significantly elevated in CPZ/Rap mice (Figures S4E and S4F), while PTN administration did not rescue the hippocampal damage cause by CPZ/Rap treatment (Figures S4E and S4F).
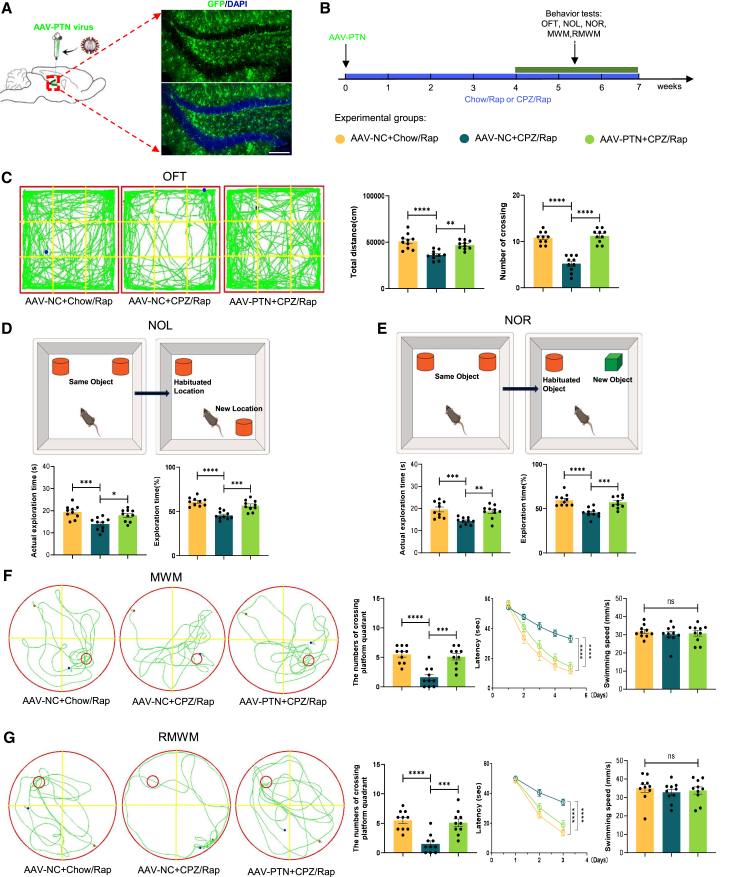
Next, we evaluated PTN’s effect on cognition through behavioral tests, including the open field test (OFT), novel object location (NOL) test, novel object recognition (NOR) test, Morris water maze (MWM), and reversal Morris water maze (RMWM). Mice were stereotaxically injected with PTN-AAV into the hippocampus and then treated with CPZ/Rap. Behavioral tests were conducted during 4–7 weeks of demyelination (Figures 5A and 5B). Consistent with previous results, CPZ/Rap mice showed a marked reduction in total distance traveled and time spent in the center zone in the OFT, indicating anxiety-like behavior (Figure 5C). CPZ/Rap mice also spent less time exploring new locations or objects in the NOL and NOR tests (Figures 5D and 5E) and had increased escape latencies and fewer platform crossings in the MWM and RMWM (Figures 5F and 5G) tests. Importantly, PTN overexpression significantly mitigated these cognitive impairments.
Figure 5.
Effects of PTN overexpression on cognitive function in CPZ/Rap-induced mice
(A and B) Schematic and timeline of experiments assessing cognitive function in CPZ/Rap mice after stereotactic injection of AAV-NC or AAV-PTN virus. Scale bars, 100 μm.
(C) Representative movement paths in the open field test (OFT) of mice injected with AAV-PTN virus post CPZ/Rap treatment. Quantification of total distance traveled and center area crossings to evaluate locomotor activities and anxiety-like behaviors. n = 10 animals per group.
(D) Schematic and quantification of the NOL test showing that PTN overexpression rescued exploratory preferences reduced by CPZ/Rap treatment. n = 10 animals per group.
(E) Schematic and quantification of the NOR test indicating that PTN-AAV overexpression rescued exploratory preferences reduced by CPZ/Rap treatment. n = 10 animals per group.
(F) Representative MWM movement paths. Quantification of platform crossing numbers during training sessions and escape latencies and swimming speed in testing sessions (two-way ANOVA: AAV-NC + Chow/Rap vs. AAV-NC + CPZ/Rap, p < 0.0001; AAV-NC + CPZ/Rap vs. PTN-AAV + CPZ/Rap, p < 0.0001). n = 10 animals per group.
(G) Representative RMWM movement paths. Quantification of platform crossing numbers during training sessions and escape latencies and swimming speed in testing sessions (two-way ANOVA: AAV-NC + Chow/Rap vs. AAV-NC+CPZ/Rap, p < 0.0001; AAV-NC + CPZ/Rap vs. PTN-AAV + CPZ/Rap, p < 0.0001). n = 10 animals per group. Data in (C–G) are presented as mean ± SEM; statistical significance was computed with one-way ANOVA and Tukey’s post hoc multiple comparisons; for (F) and (G), significance was evaluated with two-way ANOVA and Tukey’s multiple comparisons test. Non-significant comparisons are not indicated. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
In conclusion, PTN ameliorated neurogenic and cognitive deficits in the demyelinated hippocampus, although it did not enhance myelination.
PTN interacts with the PTPRZ1 or ALK receptor to regulate hippocampal neurogenesis and neuronal development in the demyelinated hippocampus
Previous studies have shown that PTN interacts with PTPRZ1 and ALK to regulate hippocampal neurogenesis and the development of newborn neurons, respectively (Li et al., 2023; Tang et al., 2019). However, whether PTN’s positive effects on NSCs and hippocampal neurons in demyelinating models are mediated through PTPRZ1 and ALK receptors remains unclear. To elucidate the roles of these receptors in demyelinated models, we engineered lentiviruses carrying short hairpin RNAs (shRNAs) against PTPRZ1 and ALK. The shRNA knockdown efficiency has been confirmed in our previous study (Tang et al., 2019).
We investigated the roles of PTPRZ1 and ALK receptors in a CPZ/Rap demyelinated model. Lentiviruses and retroviruses carrying shRNAs against PTPRZ1 and ALK were stereotaxically injected into the DG of CPZ/Rap demyelinated hippocampus, respectively, followed by PTN administration via a mini pump for 4 weeks (Figures S5A and S5B). As expected, PTPRZ1 knockdown led to a reduction in proliferating EdU+ cells, RGL NSCs, and TAP cells (Figure S5C), as well as decreased numbers of BrdU+DCX+ immature neurons (Figure S5D) and BrdU+NeuN+ mature neurons (Figure S5E). Additionally, ALK receptor knockdown resulted in developmental impairments in dendrites and dendritic spines (Figures S5F and S5G).
These findings suggest that PTN modulates NSC proliferation and differentiation via the PTPRZ1 receptor and regulates the development of hippocampal newborn neurons via the ALK receptor in the demyelinated hippocampus (Figure S5H).
Activation of AKT signaling rescues neurogenic and behavioral deficits in the demyelinated hippocampus
Our prior studies indicated that PTN cooperates with PTPRZ1 or ALK receptors to activate the AKT signaling pathway (Li et al., 2023; Tang et al., 2019). We hypothesized that the AKT activator SC79 would ameliorate neurogenic and behavioral deficits in CPZ/Rap demyelinated mice. Initially, we observed a reduction in phosphorylated AKT (p-AKT) expression in the hippocampus of CPZ/Rap mice, confirming suppression of the AKT signaling pathway during demyelination (Figure S6A). To test this hypothesis, CPZ/Rap mice were intraperitoneally injected with SC79 and subsequently underwent a series of behavioral tests and neurogenic immunostaining (Figure 6A). As expected, SC79 significantly ameliorated cognitive deficits induced by CPZ/Rap administration, as measured by the OFT, NOR, NOL, MWM, and RMWM tests (Figures S6B–S6E and 6B–6I).
Figure 6.
Effects of regulating the AKT signaling pathway on hippocampal neurogenesis, newborn neuron development, and cognitive performance in CPZ/Rap demyelinated mice
(A) Timeline of experiments investigating the role of the AKT activator SC79 in hippocampal neurogenesis, newborn neuron development, and cognitive function in CPZ/Rap mice.
(B and C) Quantification of total distance traveled and center area crossings to evaluate locomotor activities and anxiety-like behaviors in mice treated with the AKT activator (SC79) following CPZ/Rap administration in the OFT. n = 10 animals per group.
(D–I) Quantification of platform crossings in the testing session and MWM/RMWM escape latencies and swimming speed during training sessions (two-way ANOVA: for MWM test, Chow/Rap + Vehicle vs. CPZ/Rap + Vehicle, p < 0.0001, CPZ/Rap + Vehicle vs. CPZ/Rap + SC79, p < 0.0001; for RMWM test, Chow/Rap + Vehicle vs. CPZ/Rap + Vehicle, p < 0.0001, CPZ/Rap + Vehicle vs. CPZ/Rap + SC79, p < 0.01). n = 10 animals per group.
(J) Images and quantification of NSC proliferation by counting EdU+ cells, EdU+GFAP+Sox2+ RGL cells, and EdU+GFAP−Sox2+ TAP cells in the DG. n = 5 animals per group. Scale bar, 50 μm.
(K) Images and quantification of BrdU+ cells and BrdU+DCX+ immature neurons in the DG of indicated groups. n = 5 animals per group. Scale bar, 50 μm.
(L) Images and quantification of BrdU+ cells and BrdU+NeuN+ mature newborn neurons in the DG of indicated groups. n = 5 animals per group. Scale bar, 50 μm.
(M) Images and quantification of dendritic length and complexity of RFP+ newborn neurons in indicated groups (two-way ANOVA: Chow/Rap + Vehicle vs. CPZ/Rap + Vehicle, p < 0.0001; CPZ/Rap + Vehicle vs. CPZ/Rap + SC79, p < 0.0001). n = 30 neurons per group from 5 mice. Scale bar, 50 μm.
(N) Images and quantification of dendritic spine density in newborn neurons. n = 30 neurons per group from 5 mice. Scale bars, 5 μm. Data in (B–N) are presented as mean ± SEM; for (B–D), (F), (G), and (I–N), statistical significance was computed with one-way ANOVA and Tukey’s post hoc multiple comparisons; for (E), (H), and (M), significance was evaluated with two-way ANOVA. Non-significant comparisons are not indicated. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
Additionally, SC79 treatment reversed the negative effects on NSC proliferation and differentiation in CPZ/Rap mice. This resulted in an increased number of proliferating EdU+ cells, RGL cells, TAP cells, BrdU+DCX+ immature neurons, and BrdU+NeuN+ mature neurons (Figures 6J–6L). Remarkably, SC79 also rescued the developmental abnormalities of newborn neurons in CPZ/Rap mice compared to controls (Figures 6M and 6N). In summary, these results demonstrate that activation of the AKT signaling pathway through SC79 significantly ameliorates neurogenic and behavioral deficits in the CPZ/Rap demyelinated model.
To elucidate the role of PTN in the AKT signaling pathway, we used AKT inhibitor (MK2206) for CPZ/Rap mice with PTN mini-pump (Figure S6F). As expected, MK2206 administration reversed the positive effects of PTN on NSC proliferation (Figure S6G), NSC differentiation (Figures S6H and S6I), and development of newborn neurons (Figures S6J and S6K). Altogether, the PTN-AKT pathway is crucial for hippocampal neurogenesis and cognition in the CPZ/Rap demyelinated mice.
Discussion
Cognitive and affective deficits, including disorders of memory, information processing speed, attention, and executive function, along with mood disturbances, are highly prevalent in patients with MS and tend to occur early in the disease stage (Filippi et al., 2018; Jakimovski et al., 2024). These cognitive deficits significantly impact patients’ quality of life and increase the economic burden on individuals with MS. Although disease-modifying therapies have been proven to improve symptoms and prevent disease attacks, they are less effective in cognitive rehabilitation (Freedman et al., 2020). While published studies have explored the potential link between cognitive impairment and MS, the precise mechanisms of cognitive decline in MS remain unknown. Therefore, our focus is on uncovering the underlying mechanisms of cognitive deficits in MS for better disease management.
PTN, a neurotrophic and neuroprotective factor, has been confirmed to be elevated in various cancers and neurodegenerative and neuroinflammatory diseases, where it inhibits disease progression (Pufe et al., 2003; Reyes-Mata et al., 2022; Skillback et al., 2017; Wang et al., 2020; Zhang et al., 2024). Several studies have indicated that PTN also participates in the regulation of cognitive function (Li et al., 2023; Skillback et al., 2017). We concentrated on the expression level and role of PTN in MS. Using single-cell RNA sequencing data, we performed bioinformatics analysis and observed increased PTN expression in astrocytes from patients with MS compared to controls (Wheeler et al., 2020). Consistent with previous findings (Reyes-Mata et al., 2022), we observed elevated concentrations of PTN in the serum and CSF of patients with MS, as measured by ELISA. Furthermore, we conducted correlation analyses between serum PTN levels and cognitive and affective scores, finding that PTN was positively correlated with cognitive scale scores and negatively correlated with anxiety and depression scores. These results suggest that PTN plays a protective role in MS.
The DG of the hippocampus is involved in learning and memory functions, as well as the regulation of anxiety and depression. AHN is a long-term process where NSCs self-renew and continuously develop into newborn neurons (Anacker and Hen, 2017; Rocca et al., 2018). AHN primarily occurs in the DG of the hippocampus and the subventricular zone (Bond et al., 2015; Guo et al., 2012). AHN in the DG plays a critical role in NSC renewal, newborn neuronal development, and the integration of hippocampal circuits, contributing to cognitive processes and affective states (Anacker and Hen, 2017; Rocca et al., 2018). Aberrant neurogenesis has been reported in several neurodegenerative diseases and psychiatric disorders, indicating a potential association between AHN and human diseases (Goncalves et al., 2016; Winner and Winkler, 2015). We focused on whether AHN is altered in MS and its link to MS-related cognitive impairment.
Pathological features of MS include demyelination, neuronal injury, and astrocyte activation (Filippi et al., 2018; Jakimovski et al., 2024; Rocca et al., 2018). Hippocampal demyelination is common in MS and correlates with cognitive decline, depression, and anxiety (Di Filippo et al., 2018; Dutta et al., 2011). Functional connectivity abnormalities of the hippocampus are detected by functional magnetic resonance imaging early in MS (Di Filippo et al., 2018; Roosendaal et al., 2010). Reduced neuronal counts and synaptic densities in the demyelinated hippocampus from MS or CPZ animal models negatively impact cognition and mood (Di Filippo et al., 2018; Dutta et al., 2011; Zhang et al., 2020). These structural and functional alterations in the hippocampus suggest neurogenic deficits in the demyelinated hippocampus. Recent studies confirmed that hippocampal neurogenesis, newborn neuron development, and dendritic spine formation are inhibited in CPZ demyelinated mice, indicating impaired AHN in the demyelinated hippocampus (Zhang et al., 2020). Using both the CPZ/Rap animal model and the LPC demyelinated model in vivo and in vitro, we found that demyelination severely impeded hippocampal neurogenesis, newborn neuronal development, and synaptic connectivity. Specifically, the demyelinated hippocampus exhibited impaired NSC proliferation and differentiation, reduced dendritic length and complexity, and decreased spine density.
Previous studies suggested that PTN is mainly secreted by NSCs (Qin et al., 2017; Tang et al., 2019). Consistent with prior studies (Baltan et al., 2021; Linnerbauer et al., 2021; Wheeler et al., 2020), we observed extensive PTN expression in astrocytes in CPZ/Rap and LPC demyelinated models. We found that NSC-secreted PTN influenced NSC proliferation and differentiation and the development of newborn neurons (Li et al., 2023; Tang et al., 2019). However, whether astrocyte-secreted PTN plays a similar role in demyelinated models was unclear. We focused on PTN’s role in the demyelinated hippocampus and confirmed that deletion of PTN from astrocytes severely impeded hippocampal neurogenesis, newborn neuron development, and synaptic connectivity. Furthermore, astrocyte PTN deletion exacerbated neurogenic deficits caused by demyelination, which could be reversed by PTN overexpression. Restoration of PTN significantly improved cognitive and affective functions in CPZ/Rap mice. These findings suggest that PTN plays a protective role in the demyelinated hippocampus by promoting neurogenesis, neuron development, and connectivity, thereby regulating cognition and mood.
Next, we explored PTN receptors and downstream pathways. PTN attaches to several receptors, such as SDC3, PTPRZ1, ALK, and CSPG5, regulating hippocampal neurogenesis and brain homeostasis (Gonzalez-Castillo et al., 2014). Our study revealed that PTN ameliorated neurogenic defects induced by CPZ/Rap via the PTPRZ1 receptor and rescued neuron development and connectivity disorders in the demyelinated hippocampus via the ALK receptor. Our prior studies identified the AKT signaling pathway as a downstream target of PTPRZ1 and ALK (Li et al., 2023; Tang et al., 2019). Activation of the AKT signaling pathway attenuated neurogenic and cognitive impairments in the CPZ/Rap demyelinated model. Additionally, we explored the link between PTN and the myelination process, finding that PTN overexpression did not affect remyelination. Thus, PTN’s positive effects on the CPZ/Rap model are primarily through initiating and activating hippocampal neurogenesis, rather than through myelination.
In conclusion, we identified an upregulation of PTN in MS, correlating positively with cognitive and affective functions. Our study revealed the protective effects of astrocyte-derived PTN on hippocampal neurogenesis and cognition during demyelination. Regulation of the PTPRZ1/ALK-AKT signaling pathway was central to PTN’s impact on the demyelinated hippocampus. These findings provide evidence for the therapeutic potential of targeting PTN signaling to ameliorate neurogenic and cognitive deficits in MS.
Experimental procedures
Animals
C57BL/6 (wild-type, 6–8 weeks) male mice were purchased from Vital River. Ptn-cKO mice were obtained by crossing Ptnf/f mice with tamoxifen-inducible ALDH1L1-CreER mice. Hippocampal demyelination was induced using CPZ and lysophosphatidylcholine (LPC). To induce the CPZ demyelination model, mice were fed a standard chow diet containing 0.2% CPZ (Sigma-Aldrich) for 4–7 weeks, with daily intraperitoneal injections of rapamycin (Rap, 10 mg/kg/day) to prevent spontaneous remyelination. For the LPC-induced demyelination model, stereotactic injections of 1 μL of 1% LPC (Sigma-Aldrich) in 0.9% NaCl were administered into the hippocampus using coordinates relative to bregma: AP: 2.0 mm, ML: 1.7 mm, DV: 1.9 mm. All mice were bred and housed with food and water, maintained at 21°C on a 12/12-h light/dark cycle at the Guangdong Laboratory Animals Monitoring Institute. All animal experiments were conducted in accordance with guidelines and approvals of the Institutional Animal Care and Use Committee of Sun Yat-sen University.
Production of lentivirus and retrovirus
Lentivirus and retrovirus packaging were prepared by cotransfecting 293T cells with lentiviral vector plasmids (psPAX2 and pMD2.G) or retrovirus vector plasmids (CMV-GP and VSVG) using polyethyleneimine (PEI, Proteintech Group, PR40001). Four hours post transfection, the supernatant containing the transfection mixture was removed and replaced with complete medium (DMEM with 10% FBS and 1% penicillin-streptomycin). The supernatant containing the virus was collected at 48, 72, and 96 h post transfection, centrifuged at 4,000 × g, and filtered through a 0.22 μm filter to remove cellular debris. Viral particles were harvested by ultracentrifugation (Beckman Coulter SW32 Ti) for 2 h at 20,000 rpm at 4°C and stored at −80°C until further use.
EdU and BrdU incorporation
To analyze NSC proliferation in vivo, mice received EdU (100 mg/kg, i.p.) 2 h before sacrifice. Brains were collected at hippocampal levels and analyzed using an EdU Cell Proliferation Kit (Beyotime, C0078L). For labeling differentiated NSCs, mice were injected intraperitoneally with BrdU (Sigma-Aldrich, B9285) at a daily dose of 50 mg/kg for 4 consecutive days. Brains were collected at 2 and 4 weeks after BrdU injection and processed for cryosectioning and immunofluorescence staining.
Tamoxifen administration
ALDH1L1-CreER; Ptnf/f mice and control mice (Ptnf/f) received daily intragastric administrations of 30 mg/mL tamoxifen (180 mg/kg, MedChem Express, HY-13757A) dissolved in corn oil for 5 consecutive days.
Stereotactic injection of mouse hippocampus
Mice were anesthetized, followed by LPC and viruses with titers greater than 10ˆ8/mL injected into the bilateral hippocampus using stereotaxic equipment (RWD Life Science, Shenzhen, China). The injection site was determined according to the brain map (coordinates relative to bregma: AP: 2.0 mm, ML: 1.7 mm, DV: 1.9 mm). Mice were placed on a thermostatic heat pad after the stereotactic injection surgery and then returned to their home cages.
Statistical analysis
All analyses were carried out using GraphPad Prism version 9. Results are presented as mean ± SEM at least triplicate technical replicates. Statistical analysis was performed using an unpaired, one-tailed Student’s t test, one-way ANOVA, or two-way ANOVA with Tukey’s post hoc test. Statistical significance between groups was set at p < 0.05.
Additional procedures
Please see supplemental document for additional experimental procedure information.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Changyong Tang (tangchy23@mail.sysu.edu.cn).
Materials availability
All materials and lines generated in this study are available from the lead contact.
Data and code availability
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
We thank Weixiang Guo, Xiusheng Qiu, Caixia Wu, Peijian Peng, and Min Yang for helpful discussion and technical assistance. This research was supported by grants from the Science and Technology Plan Project of Guangzhou City (grant 2023A04J1089 to C.T.), the National Natural Science Foundation of China (grants 82471382 to C.T., 82101402 to L.Z., 82401413 to W.J., and 82101402 to L.Z.), and the National Key R&D Program of China (grant 2022ZD0214300 to C.T.).
Author contributions
Conceptualization, C.T.; methodology, Y.S., H.L., W.J., Y.L., F.H.N., and H.X.; investigation, Y.S., H.L., Y.L., and W.J.; writing – original draft, Y.S.; writing – review and editing, C.T. and Y.S.; funding acquisition, C.T., L.Z., and W.J.; supervision, C.T.; project administration, C.T.
Declaration of interests
The authors declare no competing interests.
Published: December 26, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2024.11.013.
Contributor Information
Wei Jiang, Email: boyuejiangwei@163.com.
Lu Zheng, Email: zhenglu6@mail.sysu.edu.cn.
Changyong Tang, Email: tangchy23@mail.sysu.edu.cn.
Supplemental information
References
- Adebiyi O.E., Bynoe M.S. Roles of Adenosine Receptor (subtypes A(1) and A(2A)) in Cuprizone-Induced Hippocampal Demyelination. Mol. Neurobiol. 2023;60:5878–5890. doi: 10.1007/s12035-023-03440-6. [DOI] [PubMed] [Google Scholar]
- Anacker C., Hen R. Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nat. Rev. Neurosci. 2017;18:335–346. doi: 10.1038/nrn.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltan S., Jawaid S.S., Chomyk A.M., Kidd G.J., Chen J., Battapady H.D., Chan R., Dutta R., Trapp B.D. Neuronal hibernation following hippocampal demyelination. Acta Neuropathol. Commun. 2021;9:34. doi: 10.1186/s40478-021-01130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict R.H.B., Amato M.P., DeLuca J., Geurts J.J.G. Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020;19:860–871. doi: 10.1016/S1474-4422(20)30277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer F., Lüdje W., Karpf J., Saher G., Beckervordersandforth R. Distribution of Aldh1L1-CreER(T2) Recombination in Astrocytes Versus Neural Stem Cells in the Neurogenic Niches of the Adult Mouse Brain. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.713077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond A.M., Ming G.L., Song H. Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later. Cell Stem Cell. 2015;17:385–395. doi: 10.1016/j.stem.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Filippo M., Portaccio E., Mancini A., Calabresi P. Multiple sclerosis and cognition: synaptic failure and network dysfunction. Nat. Rev. Neurosci. 2018;19:599–609. doi: 10.1038/s41583-018-0053-9. [DOI] [PubMed] [Google Scholar]
- Dutta R., Chang A., Doud M.K., Kidd G.J., Ribaudo M.V., Young E.A., Fox R.J., Staugaitis S.M., Trapp B.D. Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Ann. Neurol. 2011;69:445–454. doi: 10.1002/ana.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M., Bar-Or A., Piehl F., Preziosa P., Solari A., Vukusic S., Rocca M.A. Multiple sclerosis. Nat. Rev. Dis. Primers. 2018;4:43. doi: 10.1038/s41572-018-0041-4. [DOI] [PubMed] [Google Scholar]
- Freedman M.S., Devonshire V., Duquette P., Giacomini P.S., Giuliani F., Levin M.C., Montalban X., Morrow S.A., Oh J., Rotstein D., et al. Treatment Optimization in Multiple Sclerosis: Canadian MS Working Group Recommendations. Can. J. Neurol. Sci. 2020;47:437–455. doi: 10.1017/cjn.2020.66. [DOI] [PubMed] [Google Scholar]
- Goncalves J.T., Schafer S.T., Gage F.H. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell. 2016;167:897–914. doi: 10.1016/j.cell.2016.10.021. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Castillo C., Ortuno-Sahagun D., Guzman-Brambila C., Pallas M., Rojas-Mayorquin A.E. Pleiotrophin as a central nervous system neuromodulator, evidences from the hippocampus. Front. Cell. Neurosci. 2014;8:443. doi: 10.3389/fncel.2014.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Patzlaff N.E., Jobe E.M., Zhao X. Isolation of multipotent neural stem or progenitor cells from both the dentate gyrus and subventricular zone of a single adult mouse. Nat. Protoc. 2012;7:2005–2012. doi: 10.1038/nprot.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakimovski D., Bittner S., Zivadinov R., Morrow S.A., Benedict R.H., Zipp F., Weinstock-Guttman B. Multiple sclerosis. Lancet. 2024;403:183–202. doi: 10.1016/S0140-6736(23)01473-3. [DOI] [PubMed] [Google Scholar]
- Krellman J.W., Ruiz H.H., Marciano V.A., Mondrow B., Croll S.D. Behavioral and neuroanatomical abnormalities in pleiotrophin knockout mice. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Xu L., Jiang W., Qiu X., Xu H., Zhu F., Hu Y., Liang S., Cai C., Qiu W., et al. Pleiotrophin ameliorates age-induced adult hippocampal neurogenesis decline and cognitive dysfunction. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.113022. [DOI] [PubMed] [Google Scholar]
- Linnerbauer M., Lößlein L., Farrenkopf D., Vandrey O., Tsaktanis T., Naumann U., Rothhammer V. Astrocyte-Derived Pleiotrophin Mitigates Late-Stage Autoimmune CNS Inflammation. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.800128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnerbauer M., Wheeler M.A., Quintana F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron. 2020;108:608–622. doi: 10.1016/j.neuron.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Mashour G.A., Webster H.F., Kurtz A. Basic FGF and FGF receptor 1 are expressed in microglia during experimental autoimmune encephalomyelitis: temporally distinct expression of midkine and pleiotrophin. Glia. 1998;24:390–397. doi: 10.1002/(sici)1098-1136(199812)24:4<390::aid-glia4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Ming G.L., Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos D., Dukes S., Patel R., Nicholas R., Vora A., Reynolds R. Substantial archaeocortical atrophy and neuronal loss in multiple sclerosis. Brain Pathol. 2009;19:238–253. doi: 10.1111/j.1750-3639.2008.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planche V., Gibelin M., Cregut D., Pereira B., Clavelou P. Cognitive impairment in a population-based study of patients with multiple sclerosis: differences between late relapsing-remitting, secondary progressive and primary progressive multiple sclerosis. Eur. J. Neurol. 2016;23:282–289. doi: 10.1111/ene.12715. [DOI] [PubMed] [Google Scholar]
- Pufe T., Bartscher M., Petersen W., Tillmann B., Mentlein R. Pleiotrophin, an embryonic differentiation and growth factor, is expressed in osteoarthritis. Osteoarthritis Cartilage. 2003;11:260–264. doi: 10.1016/s1063-4584(02)00385-0. [DOI] [PubMed] [Google Scholar]
- Qian K., Jiang X., Liu Z.Q., Zhang J., Fu P., Su Y., Brazhe N.A., Liu D., Zhu L.Q. Revisiting the critical roles of reactive astrocytes in neurodegeneration. Mol. Psychiatry. 2023;28:2697–2706. doi: 10.1038/s41380-023-02061-8. [DOI] [PubMed] [Google Scholar]
- Qin E.Y., Cooper D.D., Abbott K.L., Lennon J., Nagaraja S., Mackay A., Jones C., Vogel H., Jackson P.K., Monje M. Neural Precursor-Derived Pleiotrophin Mediates Subventricular Zone Invasion by Glioma. Cell. 2017;170:845–859.e19. doi: 10.1016/j.cell.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Mata P.M., Rojas-Mayorquín A.E., Carrera-Quintanar L., González-Castillo C., Mireles-Ramírez M.A., Guerrero-García J.d.J., Ortuño-Sahagún D. Pleiotrophin Serum Level is Increased in Relapsing-Remitting Multiple Sclerosis and Correlates With Sex, BMI and Treatment. Arch. Med. Res. 2022;53:59–68. doi: 10.1016/j.arcmed.2021.06.005. [DOI] [PubMed] [Google Scholar]
- Rocca M.A., Barkhof F., De Luca J., Frisén J., Geurts J.J.G., Hulst H.E., Sastre-Garriga J., Filippi M., MAGNIMS Study Group The hippocampus in multiple sclerosis. Lancet Neurol. 2018;17:918–926. doi: 10.1016/S1474-4422(18)30309-0. [DOI] [PubMed] [Google Scholar]
- Roosendaal S.D., Hulst H.E., Vrenken H., Feenstra H.E.M., Castelijns J.A., Pouwels P.J.W., Barkhof F., Geurts J.J.G. Structural and functional hippocampal changes in multiple sclerosis patients with intact memory function. Radiology. 2010;255:595–604. doi: 10.1148/radiol.10091433. [DOI] [PubMed] [Google Scholar]
- Skillback T., Mattsson N., Hansson K., Mirgorodskaya E., Dahlen R., van der Flier W., Scheltens P., Duits F., Hansson O., Teunissen C., et al. A novel quantification-driven proteomic strategy identifies an endogenous peptide of pleiotrophin as a new biomarker of Alzheimer's disease. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-13831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skripuletz T., Wurster U., Worthmann H., Heeren M., Schuppner R., Trebst C., Kielstein J.T., Weissenborn K., Stangel M. Blood-cerebrospinal fluid barrier dysfunction in patients with neurological symptoms during the 2011 Northern German E. coli serotype O104:H4 outbreak. Brain. 2013;136:e241. doi: 10.1093/brain/aws361. [DOI] [PubMed] [Google Scholar]
- Srinivasan R., Lu T.Y., Chai H., Xu J., Huang B.S., Golshani P., Coppola G., Khakh B.S. New Transgenic Mouse Lines for Selectively Targeting Astrocytes and Studying Calcium Signals in Astrocyte Processes In Situ and In Vivo. Neuron. 2016;92:1181–1195. doi: 10.1016/j.neuron.2016.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C., Wang M., Wang P., Wang L., Wu Q., Guo W. Neural Stem Cells Behave as a Functional Niche for the Maturation of Newborn Neurons through the Secretion of PTN. Neuron. 2019;101:32–44.e6. doi: 10.1016/j.neuron.2018.10.051. [DOI] [PubMed] [Google Scholar]
- Wang P., Mao Y.M., Zhao C.N., Wang J.B., Li X.M., Ye D.Q., Pan H.F. Association of Midkine and Pleiotrophin Gene Polymorphisms With Systemic Lupus Erythematosus Susceptibility in Chinese Han Population. Front. Immunol. 2020;11:110. doi: 10.3389/fimmu.2020.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler M.A., Clark I.C., Tjon E.C., Li Z., Zandee S.E.J., Couturier C.P., Watson B.R., Scalisi G., Alkwai S., Rothhammer V., et al. MAFG-driven astrocytes promote CNS inflammation. Nature. 2020;578:593–599. doi: 10.1038/s41586-020-1999-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.E., Featherstone D.E. Regulation of hippocampal synaptic strength by glial xCT. J. Neurosci. 2014;34:16093–16102. doi: 10.1523/JNEUROSCI.1267-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B., Winkler J. Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2015;7 doi: 10.1101/cshperspect.a021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Kim Y., Ro E.J., Ho C., Lee D., Trapp B.D., Suh H. Hippocampal Neurogenesis and Neural Circuit Formation in a Cuprizone-Induced Multiple Sclerosis Mouse Model. J. Neurosci. 2020;40:447–458. doi: 10.1523/JNEUROSCI.0866-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Zhou K., Wang Z., Liu T., Stevens L.E., Lynce F., Chen W.Y., Peng S., Xie Y., Zhai D., et al. A subpopulation of luminal progenitors secretes pleiotrophin to promote angiogenesis and metastasis in inflammatory breast cancer. Cancer Res. 2024;84:1781–1798. doi: 10.1158/0008-5472.CAN-23-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.