Abstract
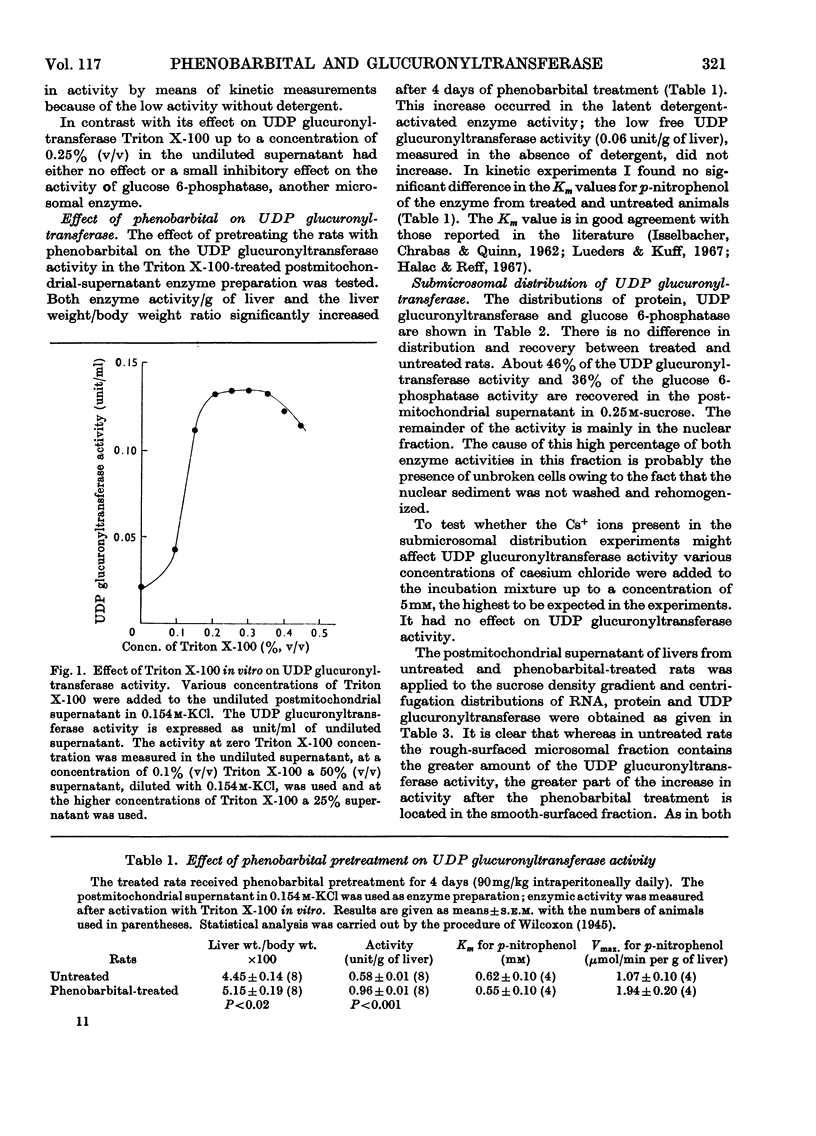
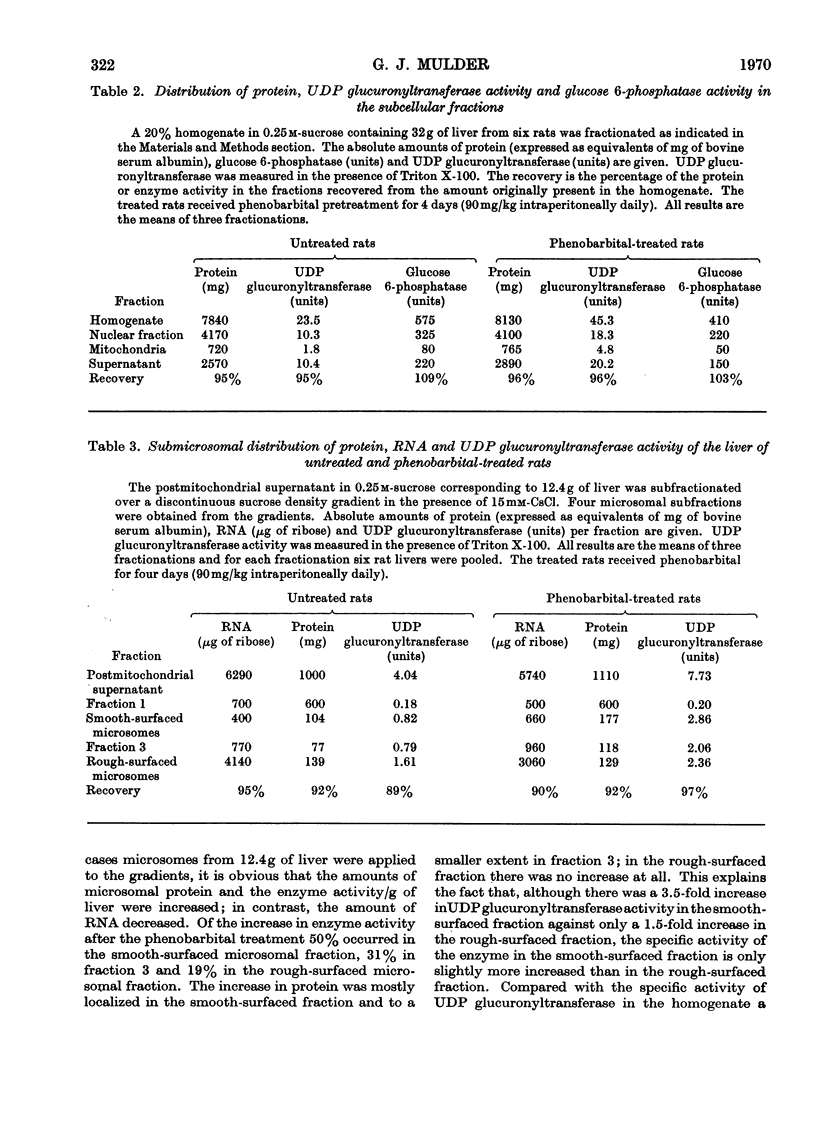
1. The detergent Triton X-100 activates UDP glucuronyltransferase from rat liver in vitro six- to seven-fold with p-nitrophenol as substrate. The enzyme activity when measured in the presence of Triton X-100 is increased significantly by pretreatment of male rats with phenobarbital for 4 days (90mg/kg each day intraperitoneally). If no Triton X-100 is applied in vitro such an increase could not be shown. In all further experiments the enzyme activity was measured after activation by Triton X-100. 2. The Km of the enzyme for the substrate p-nitrophenol does not change on phenobarbital pretreatment. 3. When the microsomal fraction from the liver of untreated rats is subfractionated on a sucrose density gradient, 47% of the enzyme activity is recovered in the rough-surfaced microsomal fraction, which also has a higher specific activity than the smooth-surfaced fraction. 4. Of the increase in activity after the phenobarbital pretreatment 50% occurs in the smooth-surfaced fraction, 19% in the rough-surfaced fraction and 31% in the fraction located between the smooth- and rough-surfaced microsomal fractions on the sucrose density gradient. 5. The latency of the enzyme in vitro, as shown by the effect of the detergent Triton X-100, is discussed in relation to the proposed heterogeneity of UDP glucuronyltransferase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Catz C., Yaffe S. J. Barbiturate enhancement of bilirubin conjugation and excretion in young and adult animals. Pediatr Res. 1968 Sep;2(5):361–370. doi: 10.1203/00006450-196809000-00005. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman P. R., Dallner G., Bergstrand A., Ernster L. Heterogeneous distribution of enzymes in submicrosomal membrane fragments. J Cell Biol. 1969 May;41(2):357–377. doi: 10.1083/jcb.41.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gram T. E., Hansen A. R., Fouts J. R. The submicrosomal distribution of hepatic uridine diphosphate glucuronyltransferases in the rabbit. Biochem J. 1968 Feb;106(3):587–591. doi: 10.1042/bj1060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARGREAVES T., LATHE G. H. INHIBITORY ASPECTS OF BILE SECRETION. Nature. 1963 Dec 21;200:1172–1176. doi: 10.1038/2001172a0. [DOI] [PubMed] [Google Scholar]
- HOLLMANN S., TOUSTER O. Alterations in tissue levels of uridine diphosphate glucose dehydrogenase, uridine diphosphate glucuronic acid pyrophosphatase and glucuronyl transferase induced by substances influencing the production of ascorbic acid. Biochim Biophys Acta. 1962 Aug 13;62:338–352. doi: 10.1016/0006-3002(62)90048-3. [DOI] [PubMed] [Google Scholar]
- Halac E., Jr, Sicignano C. Re-evaluation of the influence of sex, age pregnancy, and phenobarbital on the activity of UDP-glucuronyl transferase in rat liver. J Lab Clin Med. 1969 Apr;73(4):677–685. [PubMed] [Google Scholar]
- Henderson P. T., Dewaide J. H. Metabolism of drugs in isolated rat hepatocytes. Biochem Pharmacol. 1969 Sep;18(9):2087–2094. doi: 10.1016/0006-2952(69)90313-x. [DOI] [PubMed] [Google Scholar]
- INSCOE J. K., AXELROD J. Some factors affecting glucuronide formation in vitro. J Pharmacol Exp Ther. 1960 Jun;129:128–131. [PubMed] [Google Scholar]
- ISSELBACHER K. J., CHRABAS M. F., QUINN R. C. The solubilization and partial purification of a glucuronyl transferase from rabbit liver microsomes. J Biol Chem. 1962 Oct;237:3033–3036. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Spontaneous and detergent activation of a glucuronyltransferase in vitro. Arch Biochem Biophys. 1967 Apr;120(1):198–203. doi: 10.1016/0003-9861(67)90614-5. [DOI] [PubMed] [Google Scholar]
- SLATER T. F. Interference by sucrose in the estimation of ribonucleic acid by the orcinol method. Biochim Biophys Acta. 1958 Jan;27(1):201–202. doi: 10.1016/0006-3002(58)90311-1. [DOI] [PubMed] [Google Scholar]
- Stevenson I., Greenwood D., McEwen J. Hepatic UDP-glucuronyltransferase in Wistar and Gunn rats--in vitro activation by diethylnitrosamine. Biochem Biophys Res Commun. 1968 Sep 6;32(5):866–872. doi: 10.1016/0006-291x(68)90321-5. [DOI] [PubMed] [Google Scholar]
- Stäubli W., Hess R., Weibel E. R. Correlated morphometric and biochemical studies on the liver cell. II. Effects of phenobarbital on rat hepatocytes. J Cell Biol. 1969 Jul;42(1):92–112. doi: 10.1083/jcb.42.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidenberg P., Orrenius S., Ernster L. Increase in levels of glucuronylating enzymes and associated rise in activities of mitochondrial oxidative enzymes upon phenobarbital administration in the rat. J Cell Biol. 1967 Feb;32(2):528–531. doi: 10.1083/jcb.32.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]


