Abstract
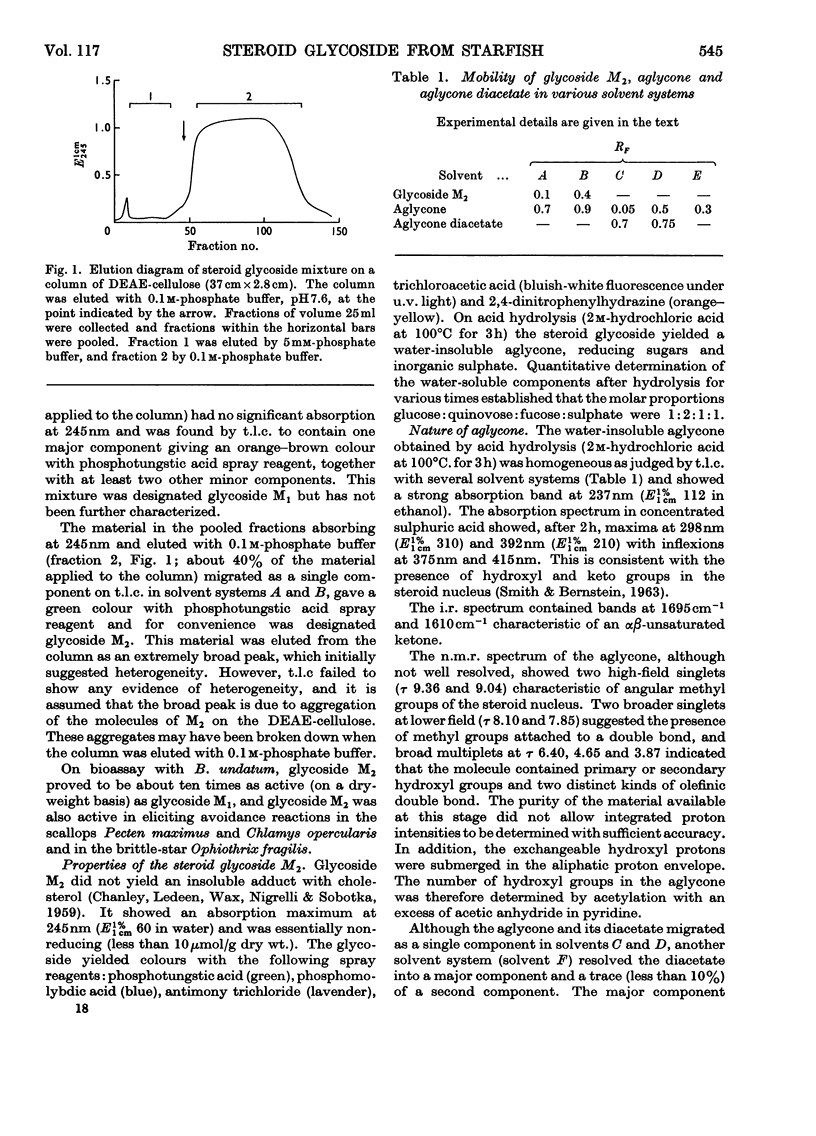
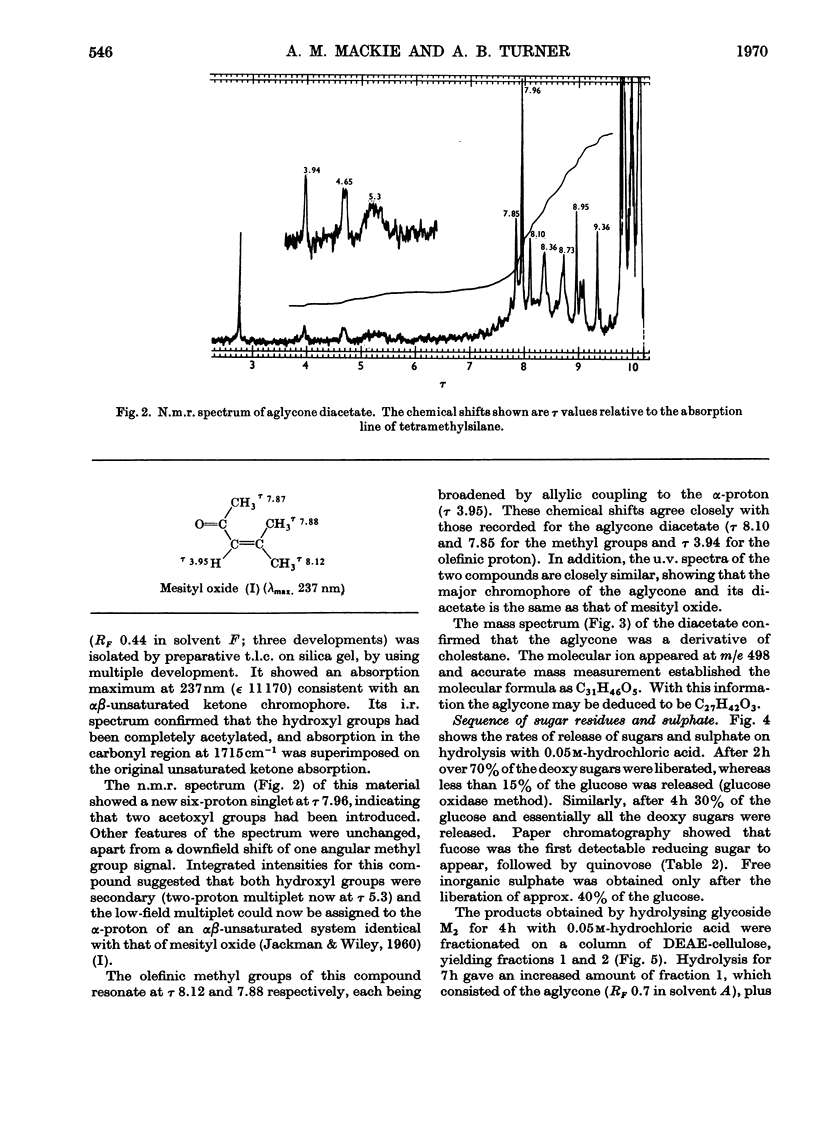
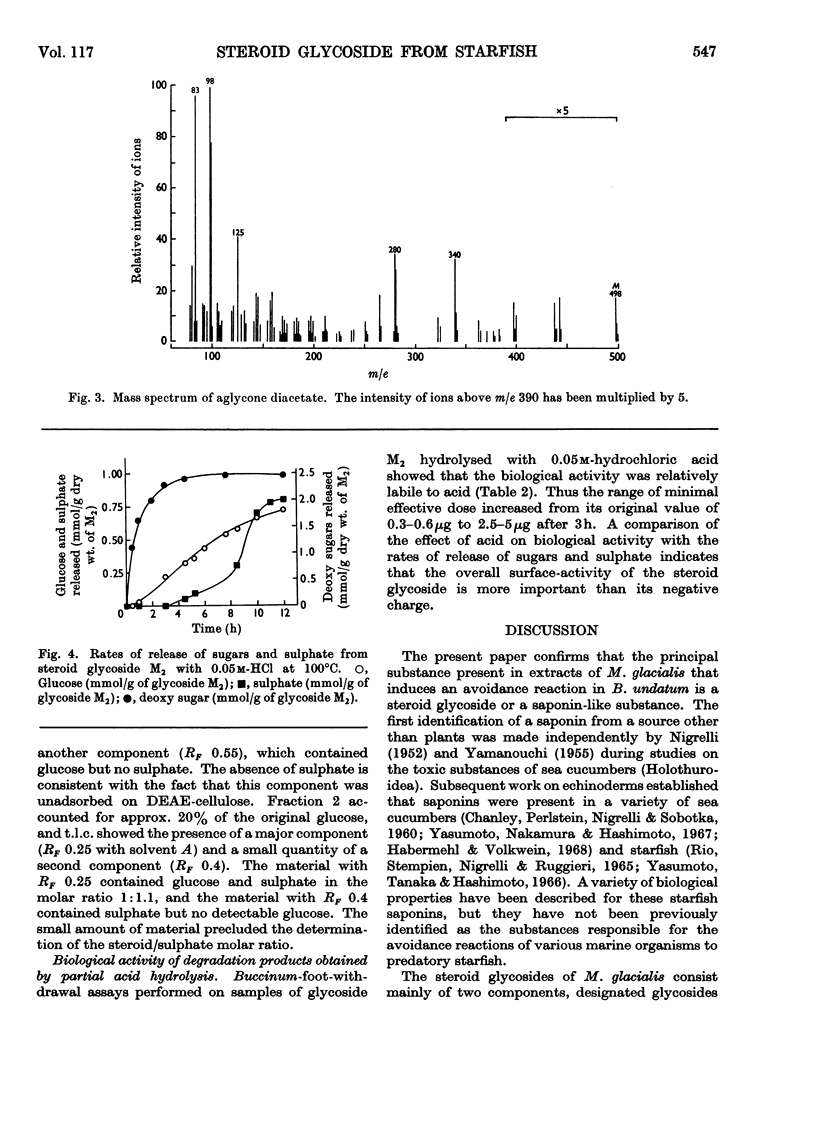
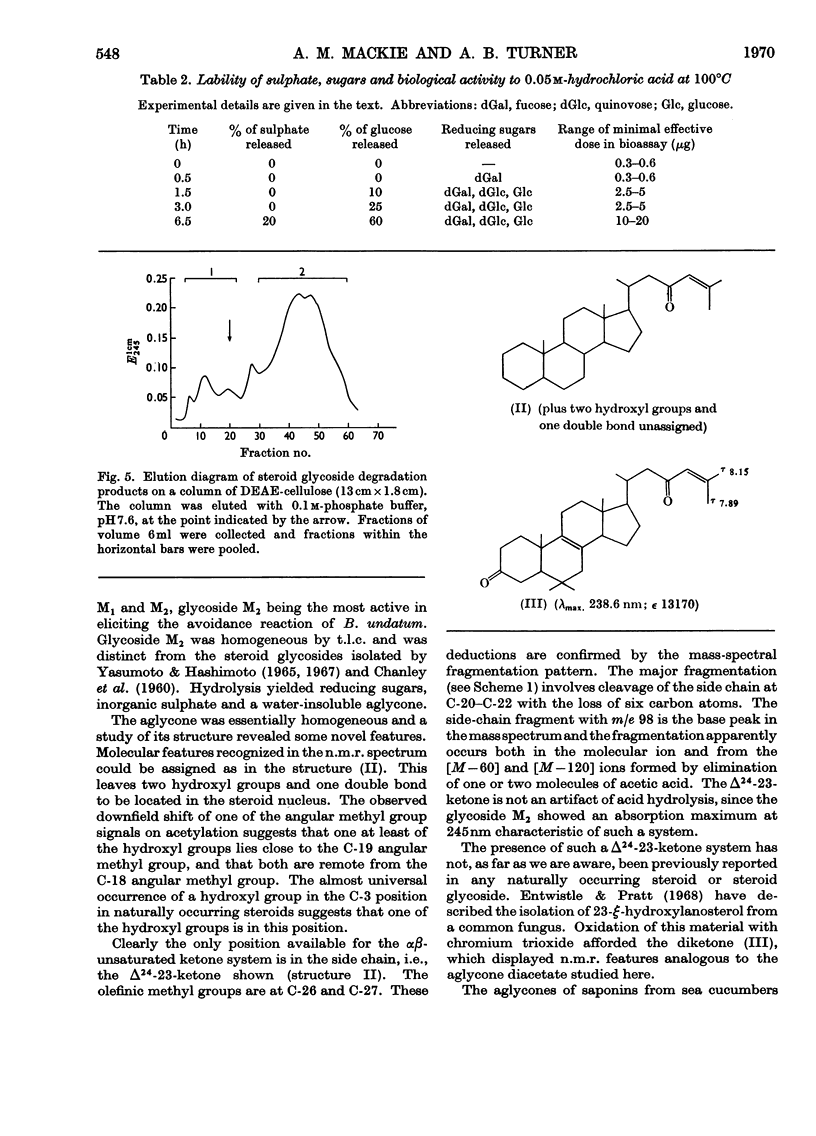
1. A steroid glycoside (M2), which induces avoidance and other reactions in the mollusc Buccinum undatum, has been isolated from extracts of the starfish Marthasterias glacialis by ion-exchange chromatography. 2. The steroid glycoside was homogeneous by t.l.c. and contained glucose, quinovose, fucose and sulphate in the molar proportions 1:2:1:1, in addition to a water-insoluble aglycone. 3. The aglycone was identified as a cholestane derivative containing an unusual Δ24-23-ketone system, two secondary hydroxyl groups and an olefinic double bond, and had the molecular formula C27H42O3. 4. The rates of release of sugars and sulphate suggested that fucose was at the non-reducing end of the oligosaccharide, with glucose glycosidically linked to the steroid. The sulphate group appeared to be linked to the other hydroxyl group of the steroid.
Full text
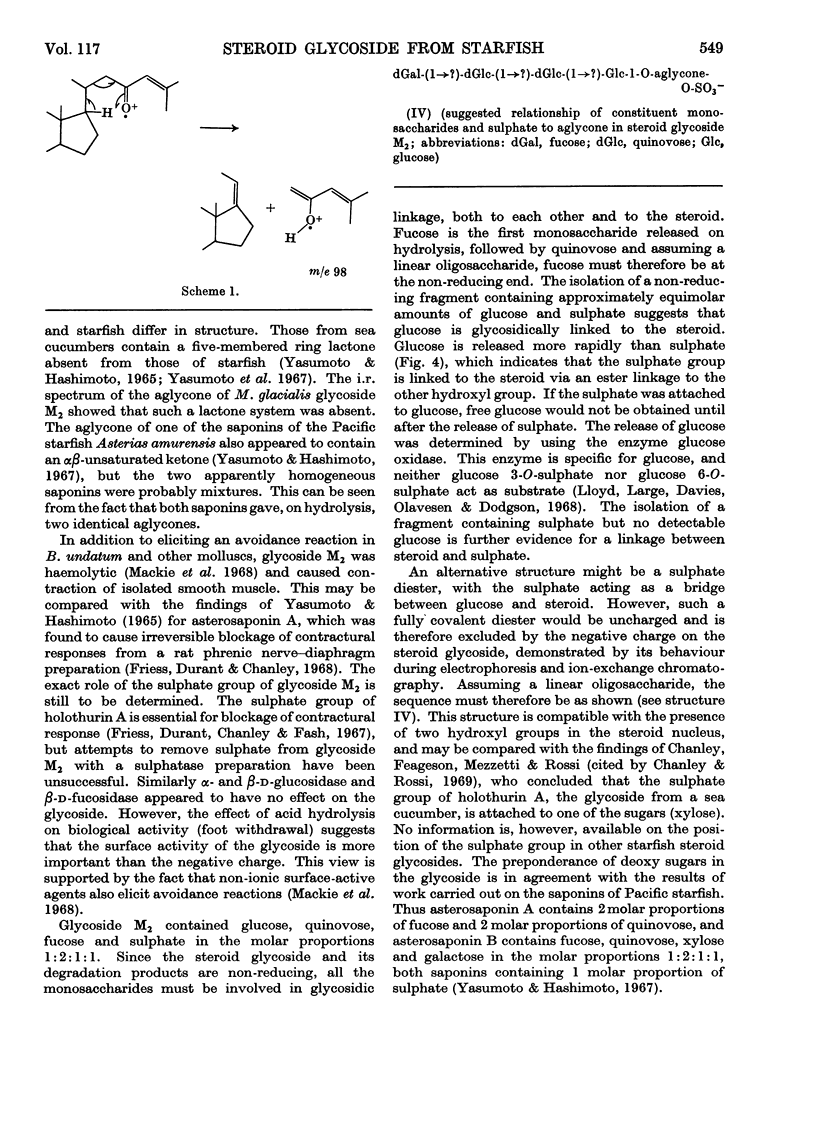
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANLEY J. D., PERISTEIN J., NIGRELLI R. F., SOBOTKA H. Further studies on the structure of holothurin. Ann N Y Acad Sci. 1960 Nov 17;90:902–905. doi: 10.1111/j.1749-6632.1960.tb26433.x. [DOI] [PubMed] [Google Scholar]
- DODGSON K. S. Determination of inorganic sulphate in studies on the enzymic and non-enzymic hydrolysis of carbohydrate and other sulphate esters. Biochem J. 1961 Feb;78:312–319. doi: 10.1042/bj0780312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friess S. L., Durant R. C., Chanley J. D., Fash F. J. Role of the sulphate charge center in irreversible interactions of holothurin A with chemoreceptors. Biochem Pharmacol. 1967 Aug;16(8):1617–1625. doi: 10.1016/0006-2952(67)90140-2. [DOI] [PubMed] [Google Scholar]
- Friess S. L., Durant R. C., Chanley J. D. Further studies on biological actions of steroidal saponins produced by poisonous echinoderms. Toxicon. 1968 Oct;6(2):81–92. doi: 10.1016/0041-0101(68)90024-x. [DOI] [PubMed] [Google Scholar]
- Habermehl G., Volkwein G. Uber Gifte der mittelmeerischen Holothurien. Naturwissenschaften. 1968 Feb;55(2):83–84. doi: 10.1007/BF00599494. [DOI] [PubMed] [Google Scholar]
- Lloyd A. G., Large P. J., Davies M., Olavesen A. H., Dodgson K. S. The glycosulphatase of Trichoderma viride. Biochem J. 1968 Jul;108(3):393–399. doi: 10.1042/bj1080393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Rio G. J., Stempien M. F., Jr, Nigrelli R. F., Ruggieri G. D. Echinoderm toxins. I. Some biochemical and physiological properties of toxins from several species of asteroidea. Toxicon. 1965 Nov;3(2):147–155. doi: 10.1016/0041-0101(65)90008-5. [DOI] [PubMed] [Google Scholar]


