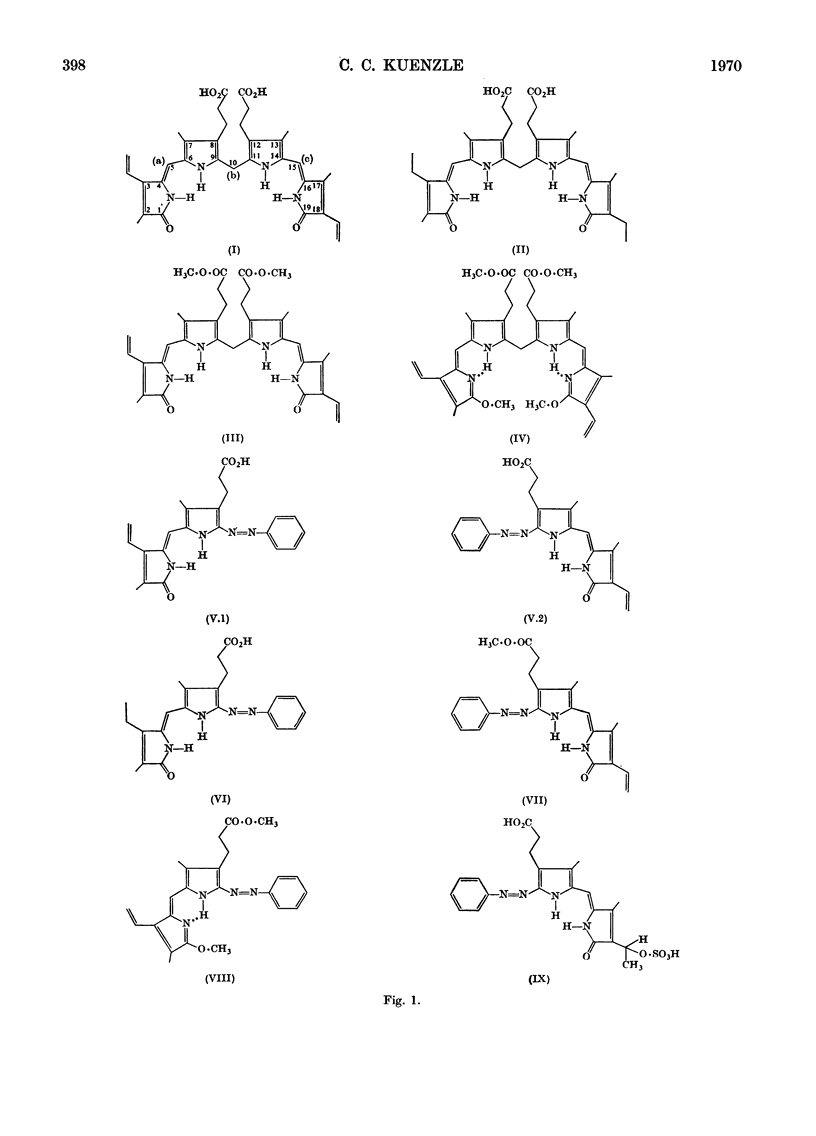
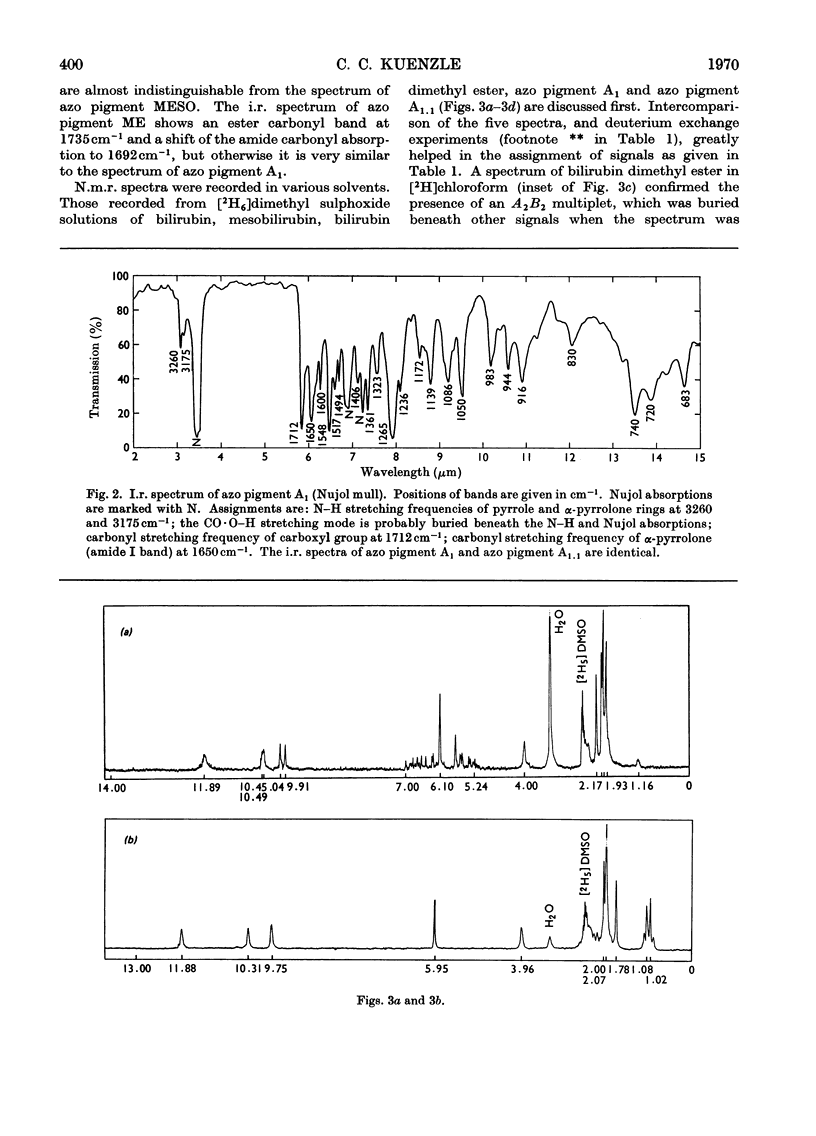
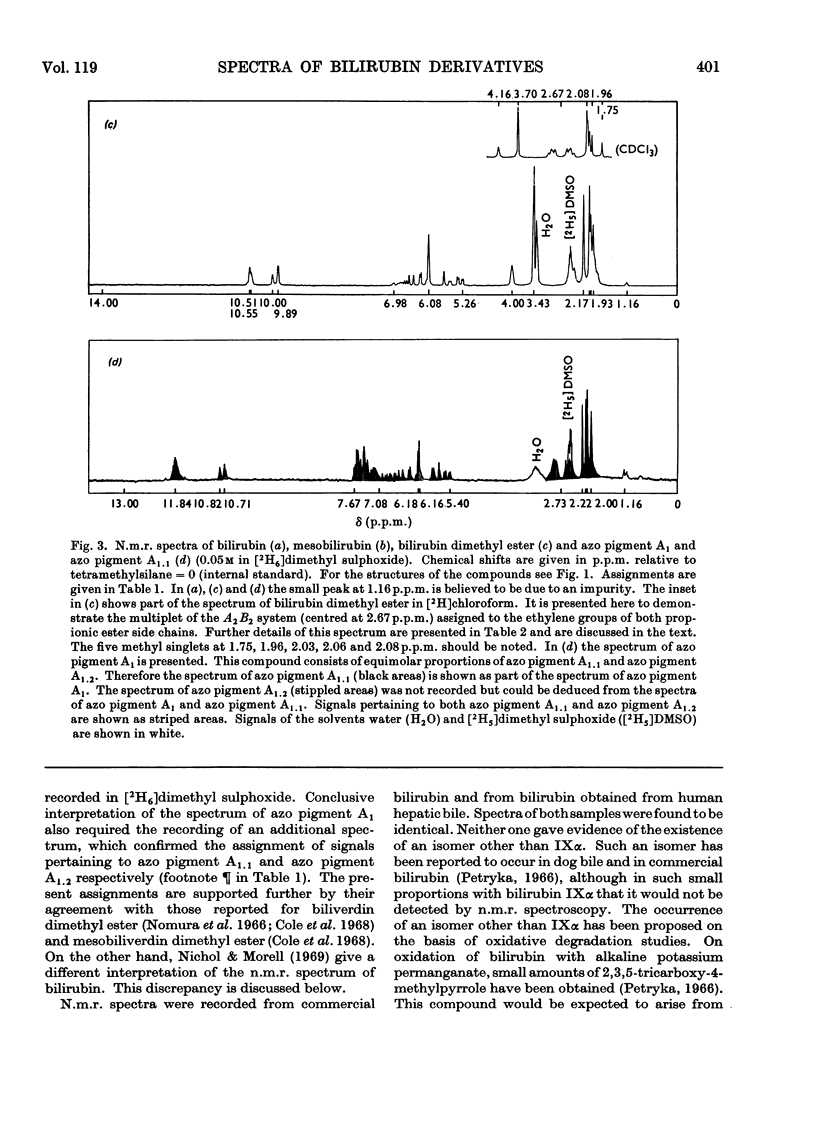
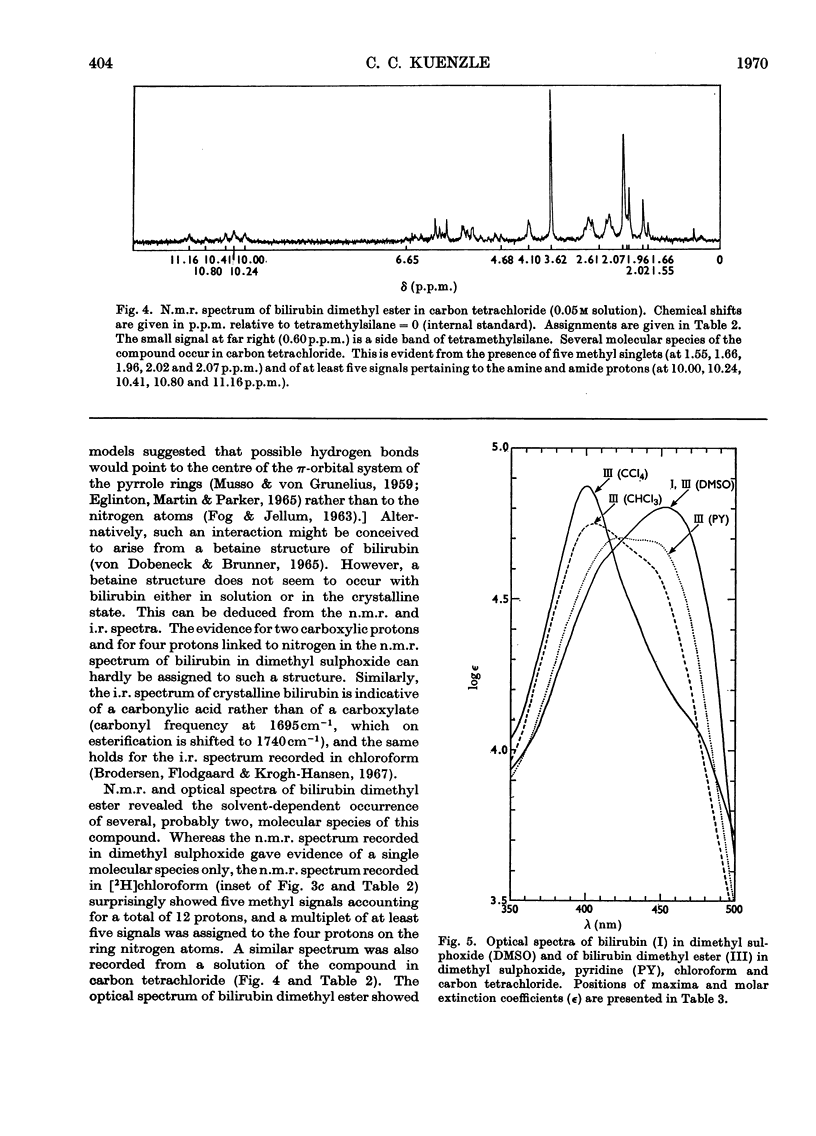
Abstract
N.m.r., i.r. and optical spectra of model compounds were recorded. These were to help in elucidating the structures of the phenylazo derivatives of bilirubin conjugates isolated from human bile. Model compounds included commercial and human bile bilirubin, mesobilirubin, bilirubin dimethyl ester, dimethoxybilirubin dimethyl ester and the corresponding phenylazo derivatives. The phenylazo derivative of vinylneoxanthobilirubinic acid was also investigated. All compounds were of the type IXα, and no other isomer could be detected with the spectroscopic methods employed. The compounds crystallize as the lactams, except for dimethoxybilirubin dimethyl ester and its phenylazo derivative, which are held in the lactim ether configuration. With all other compounds no tautomeric forms other than the lactams could be detected, although small proportions of bilirubin must exist as the lactim. Bilirubin does not form a betaine, a structure that has been proposed by von Dobeneck & Brunner (1965) to explain the bathochromic shift of its optical spectrum as compared with the expected position of the absorption maximum at 420nm. However, this shift to 453nm can be explained on the basis of internal hydrogen bonds occurring between the carboxylic protons and the pyrrole rings of bilirubin, as proposed by Fog & Jellum (1963), and new evidence for such a bonding has been accumulated. The bilirubin sulphate described by Watson (1958), which is formed by treatment of bilirubin with concentrated sulphuric acid and acetic anhydride, was also investigated. The main product of this reaction was isolated as its phenylazo derivative, and was shown to be 3,18-di(ethylidene sulphate)-2,7,13,17-tetramethylbiladiene-ac-8,12-dipropionic acid. The reaction leading to this compound is an addition of sulphuric acid to the vinyl side chains of bilirubin according to Markownikoff's rule.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brodersen R., Flodgaard H., Hansen J. K. Intramolecular hydrogen bonding in bilirubin. Acta Chem Scand. 1967;21(8):2284–2285. doi: 10.3891/acta.chem.scand.21-2284. [DOI] [PubMed] [Google Scholar]
- CLARKE J. T. PURIFICATION AND ANALYSIS OF BILIRUBIN. Clin Chem. 1965 Jul;11:681–690. [PubMed] [Google Scholar]
- Cole W. J., Chapman D. J., Siegelman H. W. The structure and properties of phycocyanobilin and related bilatrienes. Biochemistry. 1968 Aug;7(8):2929–2935. doi: 10.1021/bi00848a033. [DOI] [PubMed] [Google Scholar]
- FOG J. BILIRUBIN-PURIFICATION-PURITY. Scand J Clin Lab Invest. 1964;16:49–54. doi: 10.3109/00365516409060482. [DOI] [PubMed] [Google Scholar]
- FOG J., JELLUM E. Structure of bilirubin. Nature. 1963 Apr 6;198:88–89. doi: 10.1038/198088b0. [DOI] [PubMed] [Google Scholar]
- GRAY C. H., NICHOLSON D. C., NICOLAUS R. A. The IX-alpha structure of the common bile pigments. Nature. 1958 Jan 18;181(4603):183–185. doi: 10.1038/181183b0. [DOI] [PubMed] [Google Scholar]
- GREGORY C. H., WATSON C. J. Studies of conjugated bilirubin. II. Problem of sulfates of bilirubin in vivo and in vitro. J Lab Clin Med. 1962 Jul;60:17–30. [PubMed] [Google Scholar]
- HENRY R. J., JACOBS S. L., CHIAMORI N. Studies on the determination of bile pigments. I. Standard of purity for bilirubin. Clin Chem. 1960 Dec;6:529–536. [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. Isolation of phenylazo derivatives of bile bilirubin. Biochem J. 1970 Sep;119(3):387–394. doi: 10.1042/bj1190387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. The excretion of bilirubin as the acyl glycosides of aldobiouronic acid, pseudoaldobiouronic acid and hexuronosylhexuronic acid, with a branched-chain hexuronic acid as one of the components of the hexuronosylhexuronide. Biochem J. 1970 Sep;119(3):411–435. doi: 10.1042/bj1190411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLOYD A. G., TUDBALL N., DODGSON K. S. Infrared studies on sulphate esters. III. O-Sulphate esters of alcohols, amino alcohols and hydroxylated amino acids. Biochim Biophys Acta. 1961 Sep 30;52:413–419. doi: 10.1016/0006-3002(61)90397-3. [DOI] [PubMed] [Google Scholar]
- NEWBOLD B. T., LEBLANC G. PHYSICAL PROPERTIES OF COMMERCIAL BILIRUBINS. Can J Biochem. 1964 Dec;42:1697–1702. doi: 10.1139/o64-180. [DOI] [PubMed] [Google Scholar]
- Nichol A. W., Morell D. B. Tautomerism and hydrogen bonding in bilirubin and biliverdin. Biochim Biophys Acta. 1969 May 6;177(3):599–609. doi: 10.1016/0304-4165(69)90325-0. [DOI] [PubMed] [Google Scholar]
- Petryka Z. J. Identification of isomers differing from 9, alpha, in the early labelled bilirubin of the bile. Proc Soc Exp Biol Med. 1966 Nov;123(2):464–466. doi: 10.3181/00379727-123-31515. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Toyoda M. Infrared spectra of bilirubin and calcium bilirubinate. Chem Pharm Bull (Tokyo) 1967 Jun;15(6):899–901. [PubMed] [Google Scholar]
- WATSON C. J. Color reaction of bilirubin with sulfuric acid: a direct diazo-reacting bilirubin sulfate. Science. 1958 Jul 18;128(3316):142–143. doi: 10.1126/science.128.3316.142. [DOI] [PubMed] [Google Scholar]
- von Dobeneck H., Brunner E. Uber eine Ordnung der Dipyrromethene und über die Betainstruktur des Bilirubins. XI. Zur stokvis-Reaktion. Hoppe Seylers Z Physiol Chem. 1965;341(4):157–166. [PubMed] [Google Scholar]


