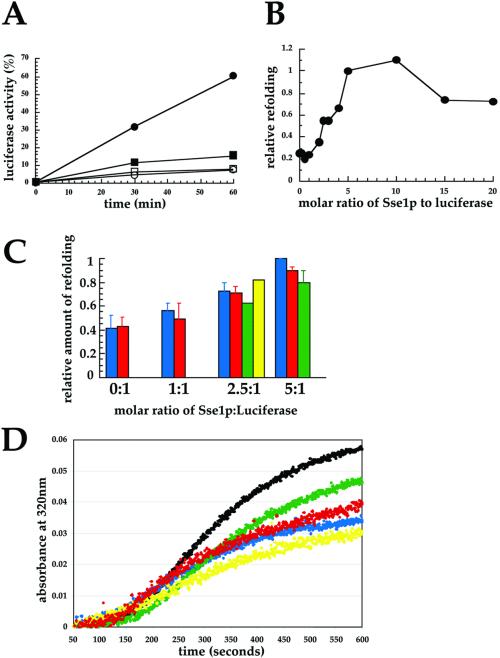
Figure 7.
Sse1p ATP-binding domain mutants are proficient for in vitro holdase activity. (A) In vitro Sse1p holdase activity was assayed as described in MATERIALS AND METHODS. ●, Reactions containing Sse1p at a 10:1 molar ratio of chaperone to luciferase during luciferase denaturation and chased with cytosol and ATP; ▪, reactions lacking Sse1p but chased with cytosol and ATP; ○, reactions containing Sse1p and chased with cytosol but no ATP; □, reactions lacking Sse1p and chased with cytosol but no ATP. Refolding activity (100%) represents the amount of luminescence when samples containing luciferase were mock denatured and then incubated with cytosol and ATP. (B) Reactions containing the indicated molar ratio of Sse1p to luciferase during thermal denaturation were chased with cytosol and ATP and luciferase refolding was assayed. The amount of refolding at a 5:1 ratio of Sse1p to luciferase was set to 1 in each experiment. Data represent the means of at least three independent experiments. (C) Relative amounts of refolding at the indicated molar ratios of wild-type Sse1p (blue), Sse1-1p (red), Sse1-2p (yellow), and the Sse1p CTD (green) to luciferase were assayed after the addition of cytosol and ATP. When error bars are present, the data represent the means of at least three independent experiments, ± SD. The amount of luciferase refolding in reactions containing a 5:1 M ratio of wild-type Sse1p to luciferase was always set to 1. (D) Luciferase aggregation over time was assayed upon thermal denaturation in the presence of a 5:1 M ratio of wild-type or the mutant Sse1p derivatives as described in MATERIALS AND METHODS. The black line indicates luciferase denatured in the absence of Sse1p, and the colored lines correspond to the protein preparations described in C.