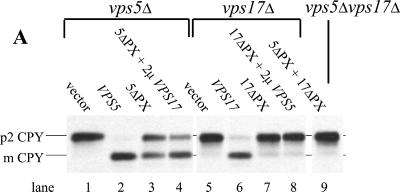
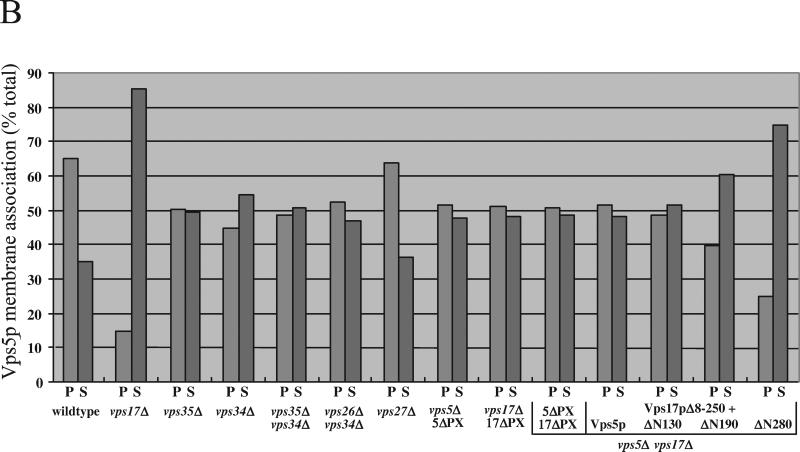
Figure 7.
(A) Deletion of the Vps5p and Vps17p PX domains exhibit different CPY sorting activity. Cells expressing the PX domain deletions with subjected to the whole cell CPY sorting assay. The 5ΔPX construct retained 50% CPY sorting activity, whereas the 17ΔPX mutant was unable to complement the CPY sorting defect in vps17Δ cells. (B) Membrane association of Vps5p seems to require binding by Vps17p and the N-terminal half of the protein. Approximately 5 OD600 nm equivalents of spheroplasted cells were labeled with [35S]methionine, chased, and then lysed. The lysates were spun at 13,000 × g to remove larger membranes such as vacuoles, endoplasmic reticulum, and plasma membrane. The supernatant was then further centrifuged at 100,000 × g for 1 h to pellet small membranes such as endosomes, vesicles, and Golgi membranes. Proteins from the supernatant (S) and pellet (P) fractions were precipitated and a lysate generated. Vps5p was recovered from the lysate by immunoprecipitation and subjected to SDS-PAGE and fluorography. The resulting autoradiogram was scanned and quantitated using NIH Image software.