Abstract
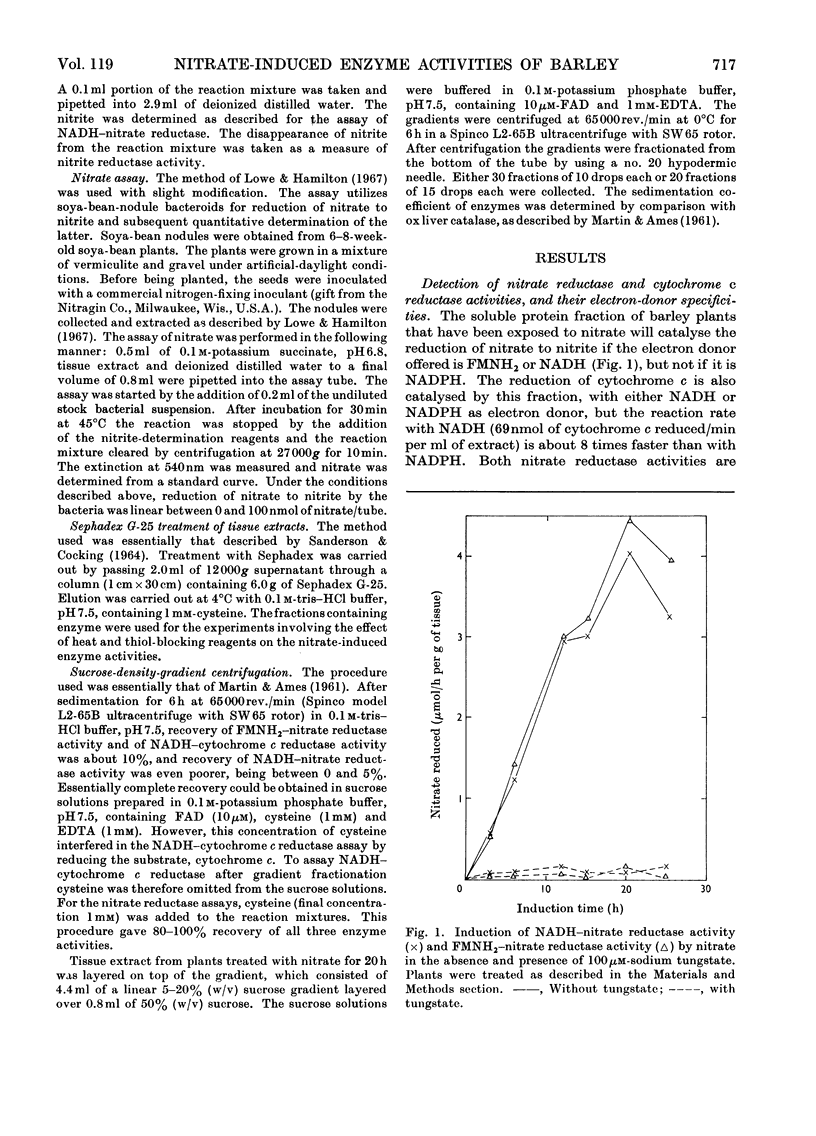
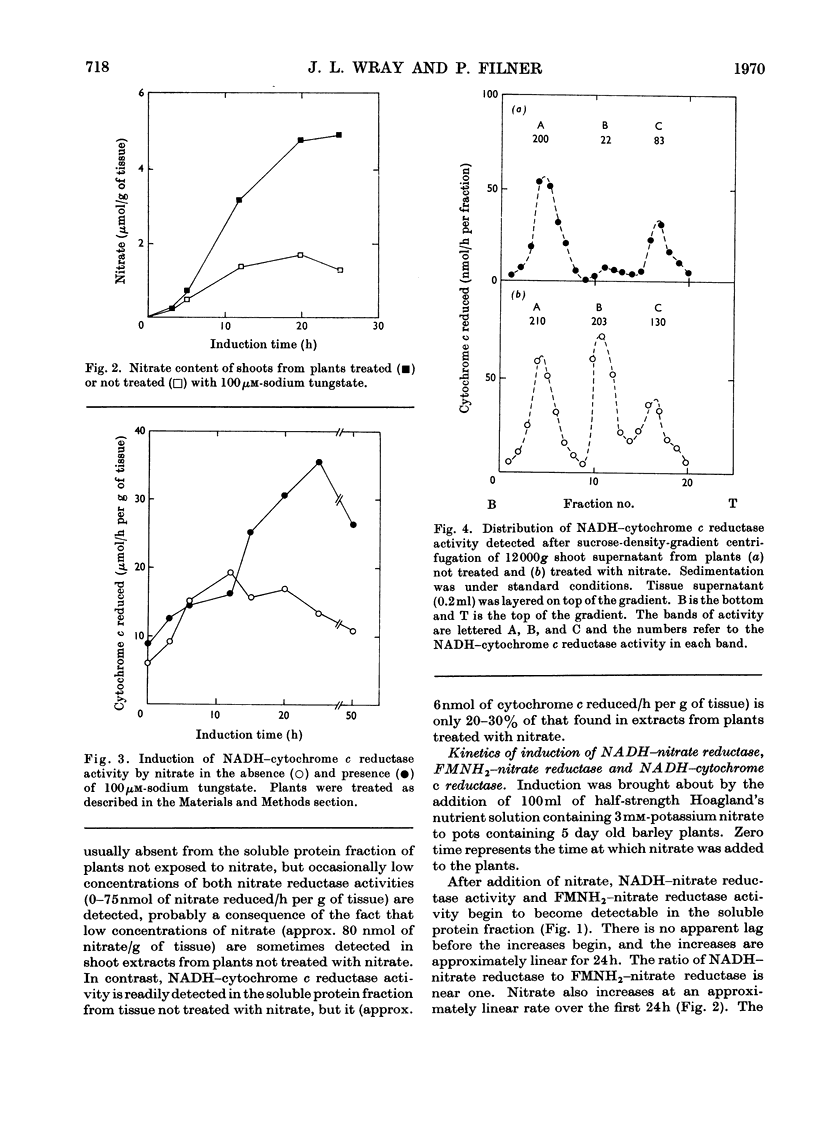
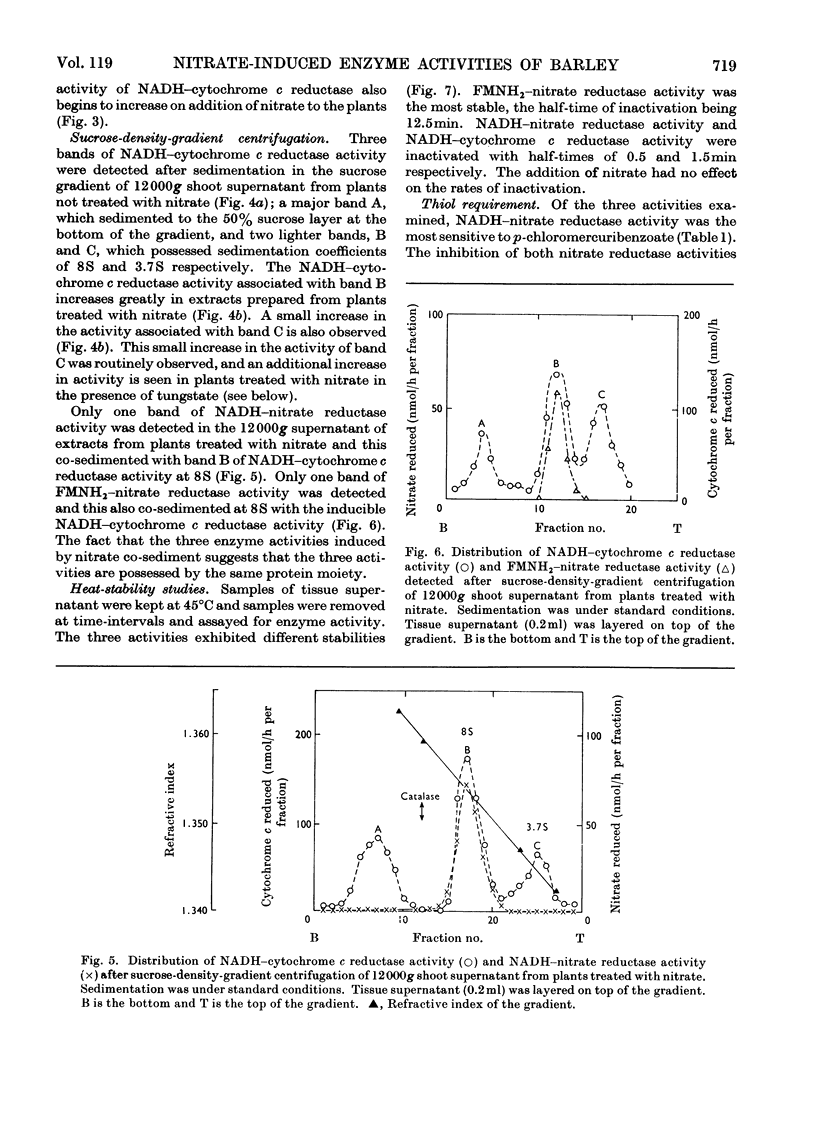
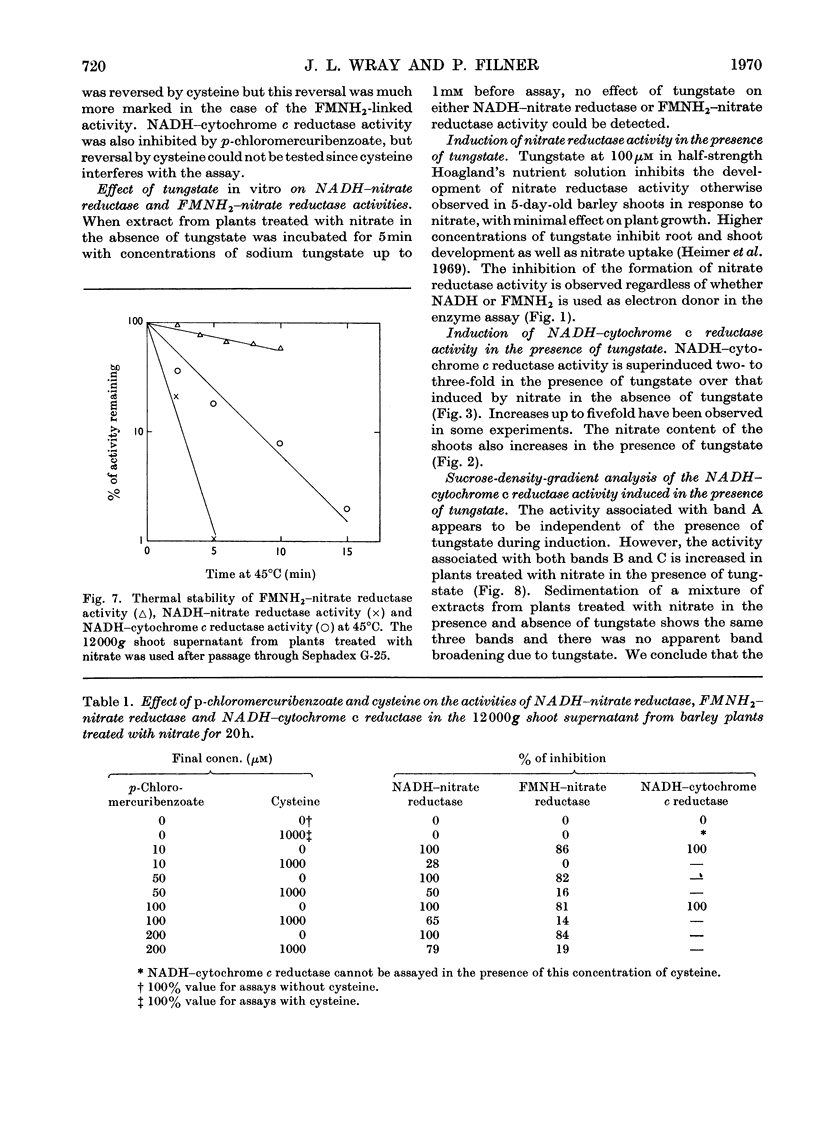
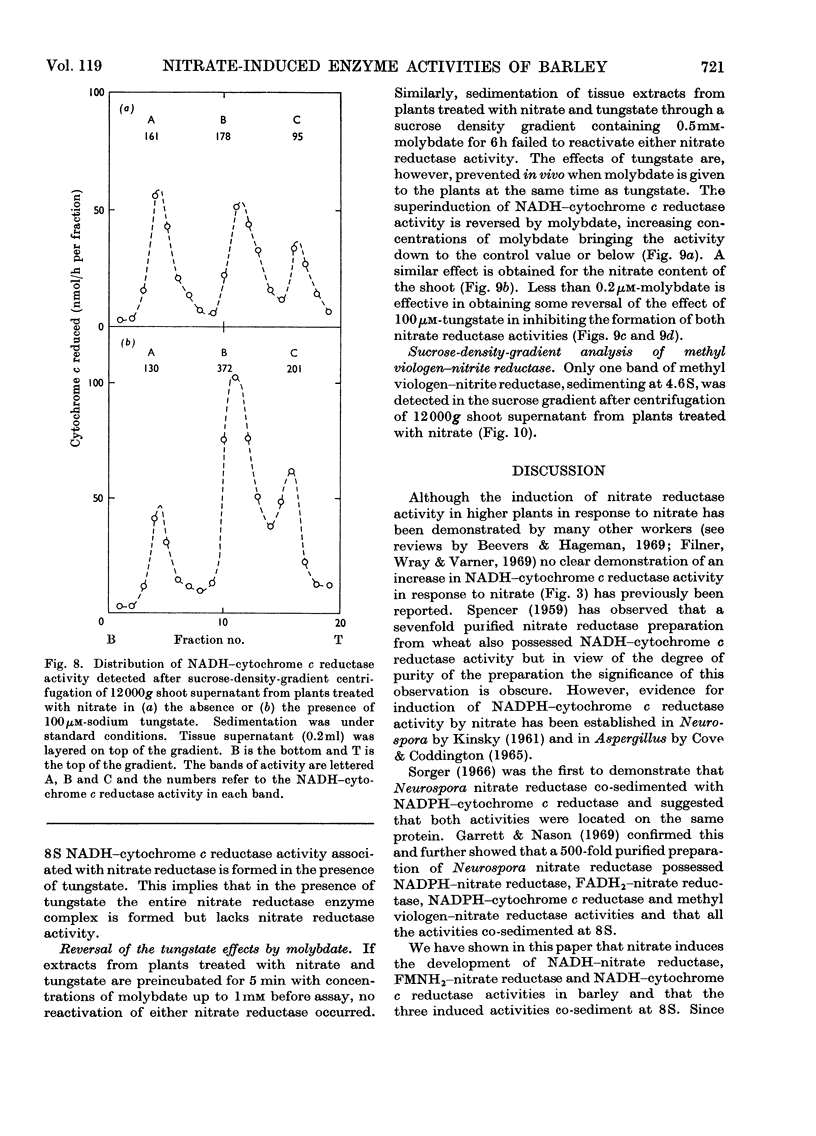
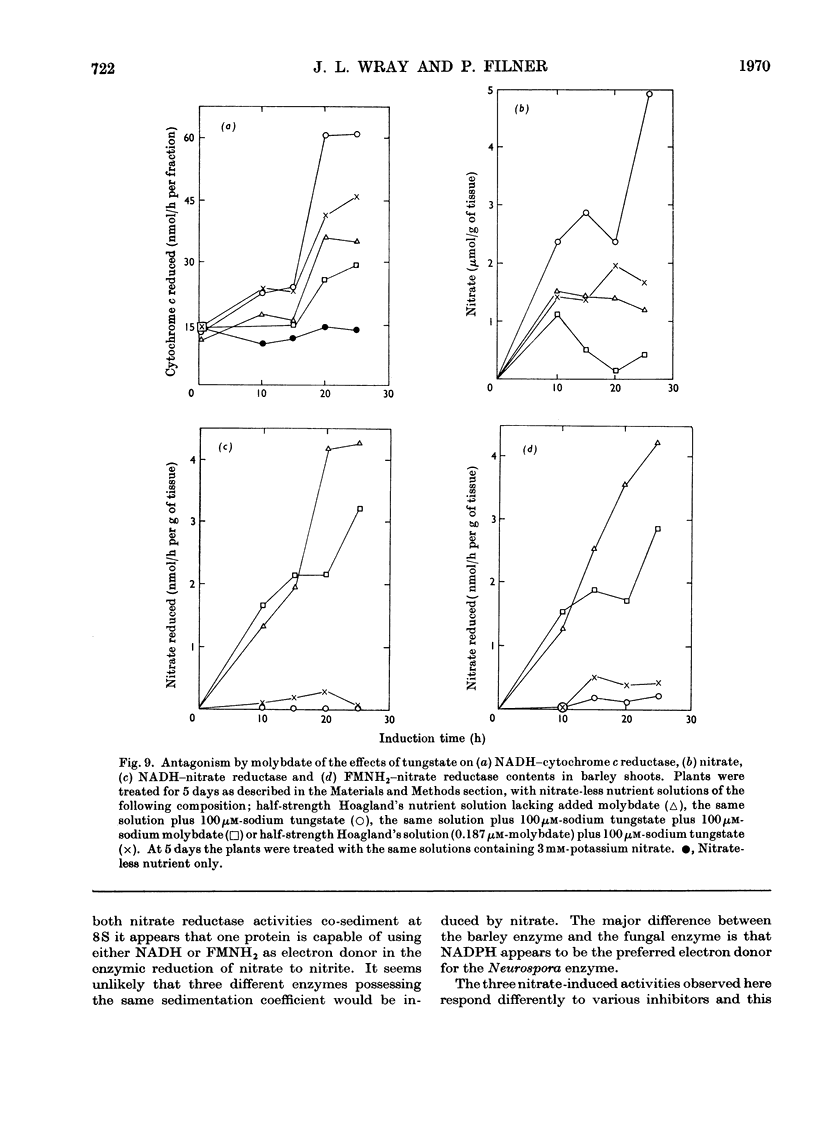
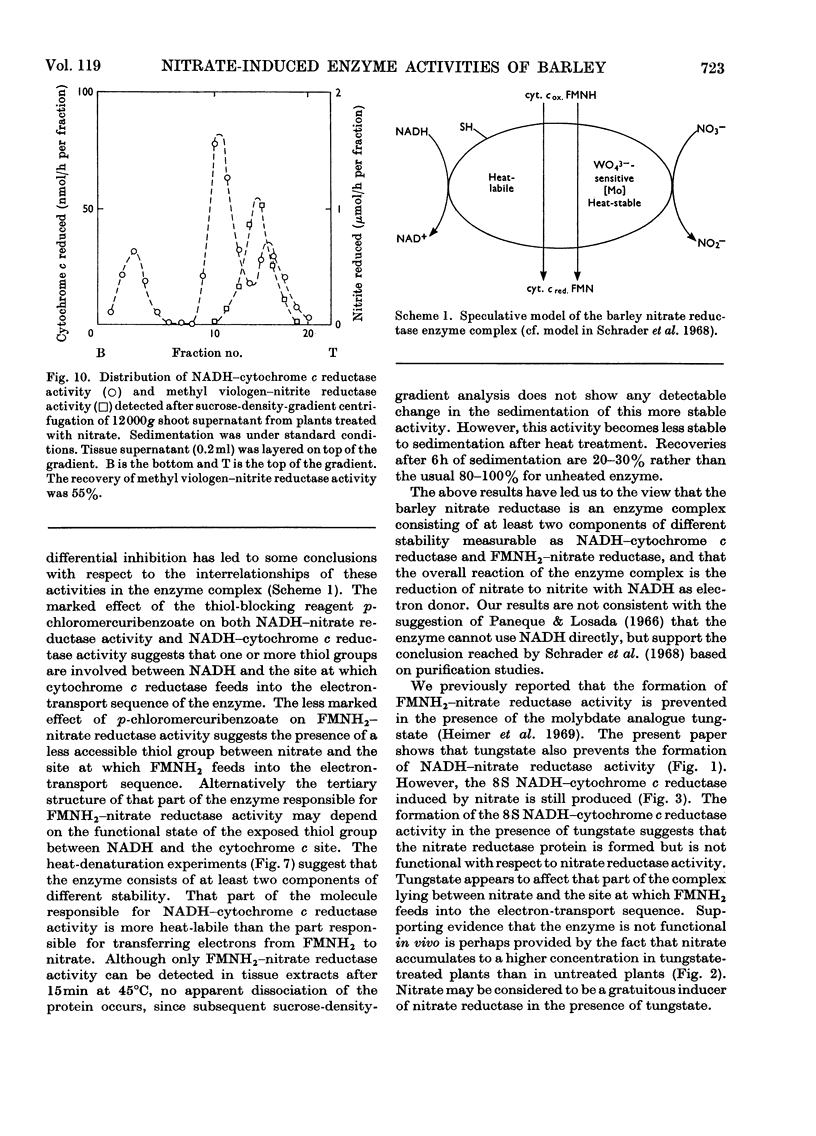
1. Nitrate induces the development of NADH-nitrate reductase (EC 1.6.6.1), FMNH2–nitrate reductase and NADH–cytochrome c reductase activities in barley shoots. 2. Sucrose-density-gradient analysis shows one band of NADH–nitrate reductase (8S), one band of FMNH2–nitrate reductase activity (8S) and three bands of NADH–cytochrome c reductase activity (bottom layer, 8S and 3.7S). Both 8S and 3.7S NADH–cytochrome c reductase activities are inducible by nitrate, but the induction of the 8S band is much more marked. 3. The 8S NADH–cytochrome c reductase band co-sediments with both NADH–nitrate reductase activity and FMNH2–nitrate reductase activity. Nitrite reductase activity (4.6S) did not coincide with the activity of either the 8S or the 3.7S NADH–cytochrome c reductase. 4. FMNH2–nitrate reductase activity is more stable (t½ 12.5min) than either NADH–nitrate reductase activity (t½ 0.5min) or total NADH–cytochrome c reductase activity (t½ 1.5min) at 45°C. 5. NADH–cytochrome c reductase and NADH–nitrate reductase activities are more sensitive to p-chloromercuribenzoate than is FMNH2–nitrate reductase activity. 6. Tungstate prevents the formation of NADH–nitrate reductase and FMNH2–nitrate reductase activities, but it causes superinduction of NADH–cytochrome c reductase activity. Molybdate overcomes the effects of tungstate. 7. The same three bands (bottom layer, 8S and 3.7S) of NADH–cytochrome c reductase activity are observed irrespective of whether induction is carried out in the presence or absence of tungstate, but only the activities in the 8S and 3.7S bands are increased. 8. The results support the idea that NADH–nitrate reductase, FMNH2–nitrate reductase and NADH–cytochrome c reductase are activities of the same enzyme complex, and that in the presence of tungstate the 8S enzyme complex is formed but is functional only with respect to NADH–cytochrome c reductase activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEEVERS L., FLESHER D., HAGEMAN R. H. STUDIES ON THE PYRIDINE NUCLEOTIDE SPECIFICITY OF NITRATE REDUCTASE IN HIGHER PLANTS AND ITS RELATIONSHIP TO SULFHYDRYL LEVEL. Biochim Biophys Acta. 1964 Sep 18;89:453–464. doi: 10.1016/0926-6569(64)90071-9. [DOI] [PubMed] [Google Scholar]
- Cove D. J., Coddington A. Purification of nitrate reductase and cytochrome c reductase from Aspergillus nidulans. Biochim Biophys Acta. 1965 Nov 22;110(2):312–318. doi: 10.1016/s0926-6593(65)80038-8. [DOI] [PubMed] [Google Scholar]
- Cove D. J., Pateman J. A. Autoregulation of the synthesis of nitrate reductase in Aspergillus nidulans. J Bacteriol. 1969 Mar;97(3):1374–1378. doi: 10.1128/jb.97.3.1374-1378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner P., Varner J. E., Wray J. L. Environmental or developmental changes cause many enzyme activities of higher plants to rise or fall. Science. 1969 Jul 25;165(3891):358–367. doi: 10.1126/science.165.3891.358. [DOI] [PubMed] [Google Scholar]
- Garrett R. H., Nason A. Further purification and properties of Neurospora nitrate reductase. J Biol Chem. 1969 Jun 10;244(11):2870–2882. [PubMed] [Google Scholar]
- Heimer Y. M., Wray J. L., Filner P. The effect of tungstate on nitrate assimilation in higher plant tissues. Plant Physiol. 1969 Aug;44(8):1197–1199. doi: 10.1104/pp.44.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle J. The regulation of activity of the enzymes involved in the assimilation of nitrate by higher plants. Biochem J. 1966 Sep;100(3):577–588. doi: 10.1042/bj1000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINSKY S. C. Induction and repression of nitrate reductase in Neurospora crassa. J Bacteriol. 1961 Dec;82:898–904. doi: 10.1128/jb.82.6.898-904.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- PATEMAN J. A., COVE D. J., REVER B. M., ROBERTS D. B. A COMMON CO-FACTOR FOR NITRATE REDUCTASE AND XANTHINE DEHYDROGENASE WHICH ALSO REGULATES THE SYNTHESIS OF NITRATE REDUCTASE. Nature. 1964 Jan 4;201:58–60. doi: 10.1038/201058a0. [DOI] [PubMed] [Google Scholar]
- Paneque A., Losada M. Comparative reduction of nitrate by spinach nitrate reductase with NADH2 and NADPH2. Biochim Biophys Acta. 1966 Oct 17;128(1):202–204. doi: 10.1016/0926-6593(66)90162-7. [DOI] [PubMed] [Google Scholar]
- Ritenour G. L., Joy K. W., Bunning J., Hageman R. H. Intracellular localization of nitrate reductase, nitrite reductase, and glutamic Acid dehydrogenase in green leaf tissue. Plant Physiol. 1967 Feb;42(2):233–237. doi: 10.1104/pp.42.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson G. W., Cocking E. C. Enzymic Assimilation of Nitrate in Tomato Plants. I. Reduction of Nitrate to Nitrite. Plant Physiol. 1964 May;39(3):416–422. doi: 10.1104/pp.39.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader L. E., Ritenour G. L., Eilrich G. L., Hageman R. H. Some characteristics of nitrate reductase from higher plants. Plant Physiol. 1968 Jun;43(6):930–940. doi: 10.1104/pp.43.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger G. J., Giles N. H. Genetic control of nitrate reductase in Neurospora crassa. Genetics. 1965 Oct;52(4):777–788. doi: 10.1093/genetics/52.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger G. J. Nitrate reductase electron transport systems in mutant and in wild-type strains of Neurospora. Biochim Biophys Acta. 1966 Jun 15;118(3):484–494. doi: 10.1016/s0926-6593(66)80091-7. [DOI] [PubMed] [Google Scholar]
- Venables W. A., Wimpenny J. W., Cole J. A. Enzymic properties of a mutant of Escherichia coli K12 lacking nitrate reductase. Arch Mikrobiol. 1968;63(2):117–121. doi: 10.1007/BF00412166. [DOI] [PubMed] [Google Scholar]


