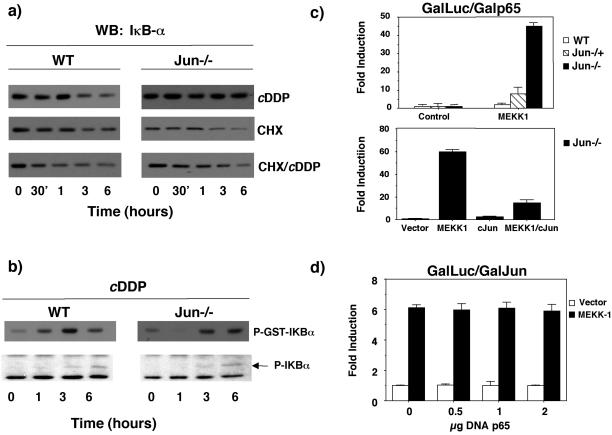
Figure 8.
c-jun inhibits p65 transcriptional activation. (a) Sustained levels of IκBα in jun−/− require de novo protein synthesis. WT and jun−/− fibroblast were preincubated during 1 h with CHX (2 μM), then treated with c-DDP, and collected after different times, as indicated. Western blots were performed using anti-IκBα antibody. (b) WT and c-jun−/− cells were stimulated with cisplatin at different times, and IKK activity was determined in an immunocomplex assay using IkBα as a substrate. Bottom: panel: the levels of phosphorylated IkBα were determined by Western blot with a specific antiphospho-IkBα antibody. (c) Top panel: WT, jun−/−, and jun−/− cells were cotransfected with a 5X-Gal-luc reporter plasmid (0.25 μg) and expression vectors encoding GAL4-p65 TAD1 (0.25 μg) and MEKK1 (0.5 μg), as indicated. After 24 h cells were collected, and relative luciferase activity was determined. Bottom panel: jun−/− cells were- cotransfected with a 5X-Gal-luc reporter plasmid (0.25 μg) and expression vectors encoding GAL4-p65 TAD1 (0.25 μg), MEKK1 (0.5 μg), and c-Jun (2 μg), as indicated. After 24 h cells were collected, and relative luciferase activity was determined. (d) Expression of p65/RelA does not inhibit c-Jun transcriptional activity. WT fibroblasts were cotransfected with a 5X-Gal-luc reporter plasmid (1 μg) and expression vectors encoding GAL4-c-Jun (1–223) (0.5 μg), MEKK1 (0.5 μg), and RC-CMVp65 (0.5–2 μg), as indicated. Luciferase activity was determined as in c. Data shown in this figure represent the mean of a single experiment performed in triplicate ±SD and are representative of at least three experiments with similar results.