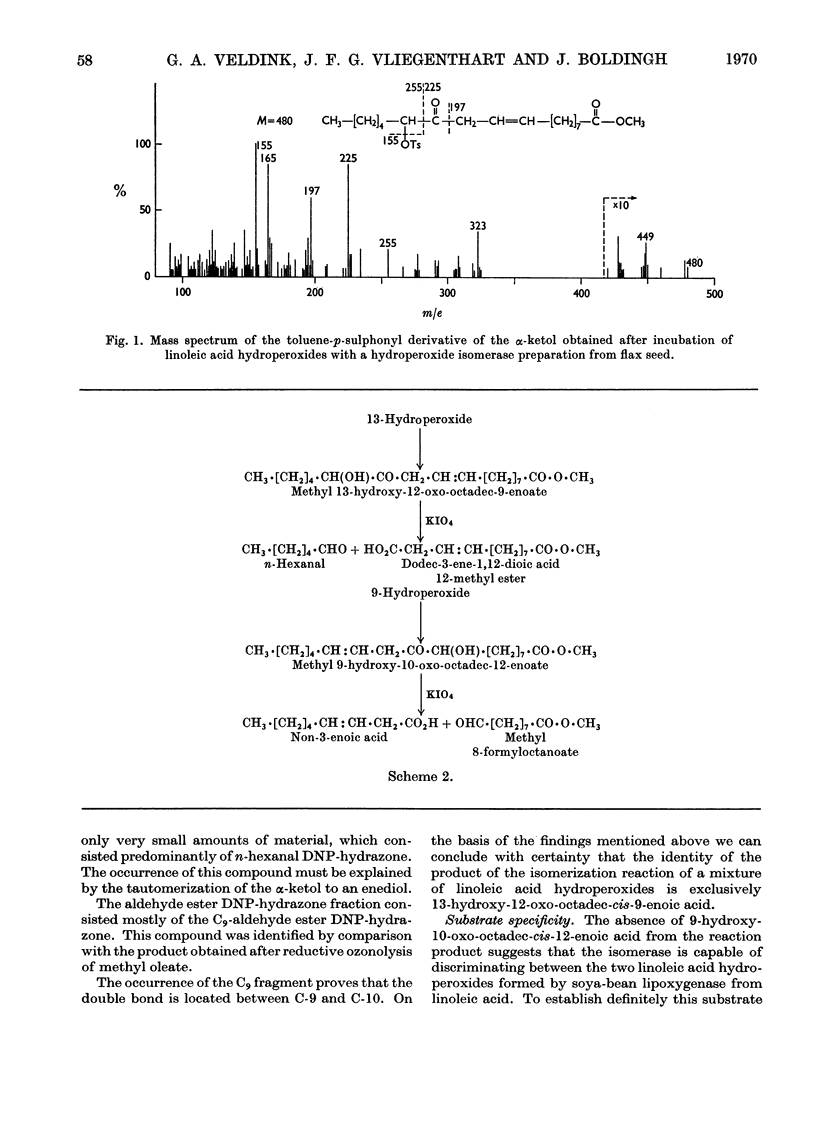
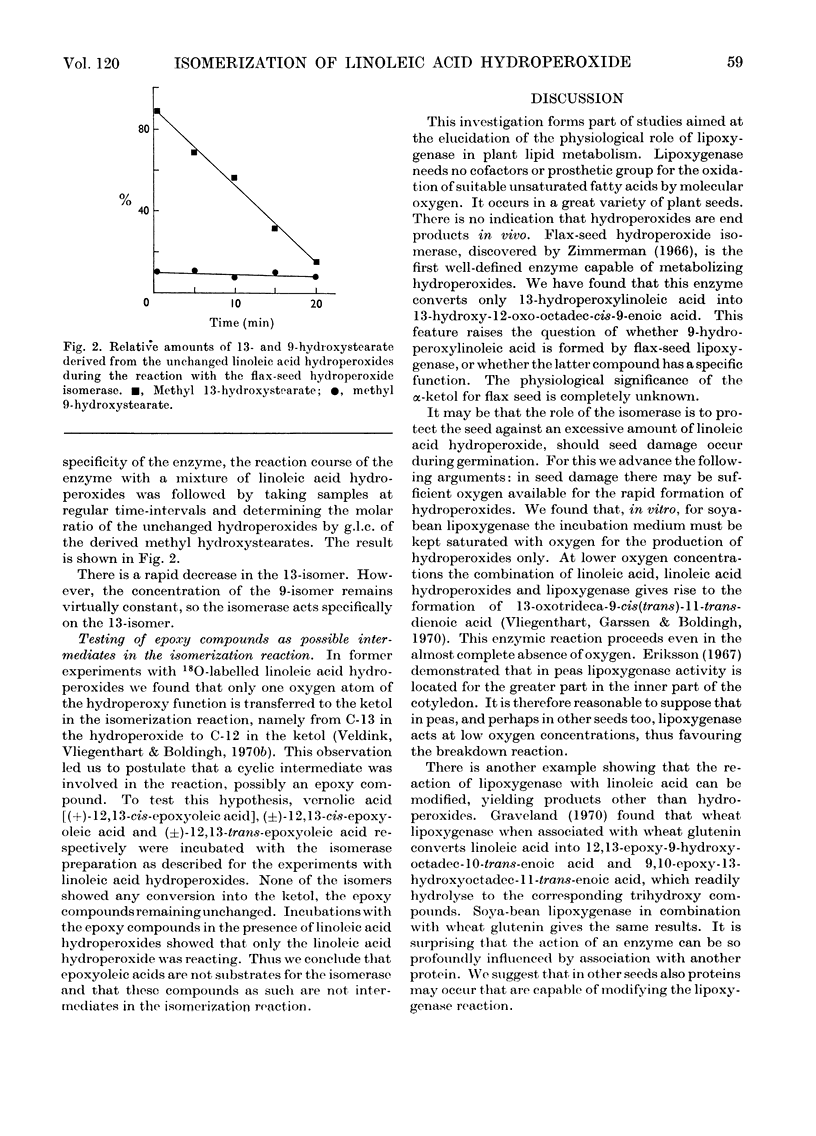
Abstract
1. The mode of action of flax-seed hydroperoxide isomerase was studied in vitro by using as substrates linoleic acid hydroperoxides formed by soya-bean lipoxygenase. 2. The enzyme converts only 13-hydroperoxyoctadeca-cis-9-trans-11-dienoic acid, whereas the 9-hydroperoxy isomer does not react. 3. The isomerization product was identified by chemical and spectroscopic methods as 13-hydroxy-12-oxo-octadec-cis-9-enoic acid. 4. 12,13-Epoxyoleic acid isomers do not act as intermediates in the isomerization reaction. 5. Suggestions for a functional relationship between hydroperoxide isomerase and lipoxygenase are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Veldink G. A., Vliegenthart J. F., Boldingh J. Proof of the enzymatic formation of 9-hydroperoxy-10-trans, 12-cis-octadecadienoic acid from linoleic acid by soya lipoxygenase. Biochim Biophys Acta. 1970 Feb 10;202(1):198–199. doi: 10.1016/0005-2760(70)90235-3. [DOI] [PubMed] [Google Scholar]
- Veldink G. A., Vliegenthart J. F., Boldingh J. The enzymic conversions of 13-hydroperoxy-cis-9-trans-11-octadecadienoic acid into 13-hydroxy-12-oxo-cis-9-octadecenoic acid. Biochem J. 1968 Dec;110(4):58P–58P. doi: 10.1042/bj1100058pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldink G. A., Vliegenthart J. F.G., Boldingh J. Oxygen transfer in the enzymatic conversion of 18O-labelled linoleic acid hydroperoxide into the 12-keto-13-hydroxyoctadec-cis-9-enoic acid. FEBS Lett. 1970 Apr 2;7(2):188–190. doi: 10.1016/0014-5793(70)80153-3. [DOI] [PubMed] [Google Scholar]
- Zimmerman D. C. A new product of linoleic acid oxidation by a flaxseed enzyme. Biochem Biophys Res Commun. 1966 May 25;23(4):398–402. doi: 10.1016/0006-291x(66)90740-6. [DOI] [PubMed] [Google Scholar]


