Abstract
Purpose
The association between second-hand smoke (SHS) exposure and cervical cancer (CC) risk is still unclear. The aim of this study is to provide an accurate and updated estimate of this association.
Methods
Through an original methodology to identify original publications, we conducted a systematic review and meta-analysis of all epidemiological studies published up to October 2022 evaluating the association between SHS exposure and CC risk among female non-smokers. Meta-analytic estimates were obtained using random-effects models and dose–response relationships were derived using log-linear functions.
Results
Out of 25 eligible studies, 21 were included in the meta-analysis, providing a pooled relative risk (RR) of cervical intraepithelial neoplasia (CIN) of grade 2 or higher of 1.52 (95% confidence interval, CI 1.30–1.78, 21 studies) for overall SHS exposure versus non-exposure. When restricting the analysis to invasive CC, the pooled RR was 1.42 (95% CI 1.17–1.71, 13 studies), whereas the pooled RR for CIN was 1.50 (95% CI 1.22–1.84, 6 studies). Analyzing RR by setting or source of SHS exposure resulted in significant associations with CC risk for SHS exposure at home (RR for CIN2+ 1.49, 95% CI 1.21–1.84, 14 studies), in non-specified settings (RR for CIN2+ 1.64, 95% CI 1.20–2.23, 8 studies) and from partner (RR for CIN2+ 1.55, 95% CI 1.25–1.94, 10 studies). The risk of CIN2+ significantly increased linearly with the intensity and pack-years of SHS exposure.
Conclusion
This comprehensive review and meta-analysis confirmed the association of SHS exposure with CC, further suggesting the need to raise concern about SHS exposure in the population.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-023-04841-9.
Keywords: Cervical cancer, Cervical intraepithelial neoplasia, Second-hand smoke, Relative risk, Meta-analysis, Dose–response relationship
Introduction
Cervical cancer (CC) is among the most common cancers worldwide, with 604,000 new cases and 342,000 deaths in 2020 (Sung et al. 2021). Human papillomavirus (HPV) infection is the leading cause of CC, with high-risk HPV persistent infections being associated with most cervical intraepithelial neoplasia (CIN) and invasive CC (Walboomers et al. 1999; Koshiol et al. 2008).
Infection from HPV is considered a necessary cause for CC (Walboomers et al. 1999) and it has been found to act together with other risk factors (Castellsagué and Muñoz 2003; Zhang et al. 2020), such as parity, oral contraceptives use, immunosuppression, and other sexually transmitted infections. In addition, a direct association between active cigarette smoking and CC risk has been established (IARC 2004; Malevolti et al. 2023).
Although the carcinogenic role of smoking on CC is well known, with a relative risk (RR) for CIN2+ from the most recent meta-analysis of 2.11 (95% CI 1.85–2.39) for current versus never smokers (Malevolti et al. 2023), studies on its indirect effect, i.e., damage from second-hand smoke (SHS) exposure, are scarce. In the comprehensive assessment of the effects of SHS carried out in 2005, the California Environmental Protection Agency (CalEPA) reported suggestive evidence of a causal association between SHS and CC, but still needing further research for confirmation (CalEPA 2005). Moreover, the 2006 report by the U.S. Public Health Service reported inadequate evidence to infer the presence or absence of a causal relationship between SHS and the risk of CC among lifetime non-smokers (U.S. Department of Health and Human Services 2006). Furthermore, in 2012 the International Agency for Research on Cancer (IARC) stated that “data are conflicting and sparse for the association of exposure to SHS with CC” (IARC 2012). Recently, a meta-analysis on 14 papers carried up to 2014 found a significant 70% increased risk of all CC (both invasive and precancerous lesions) for women exposed to SHS in comparison to non-exposed ones (Su et al. 2018). Moreover, a few studies investigated the dose–response associations with SHS exposure intensity or duration, reporting heterogeneous results (Louie et al. 2011; Hirose et al. 1996; Natphopsuk et al. 2012; Coker et al. 1992; Slattery et al. 1989; Wu et al. 2003; Min et al. 2018; Wen et al. 2022; Zhang et al. 2022). Previous studies have not considered factors that could have biased the interpretation of the analyses, such as the different definitions of non-smokers applied in each study, and the difficulty in accurately classifying the type of SHS exposure, taking into account the setting of exposure, i.e., the places where non-smokers are exposed to SHS (public spaces, workplaces, or homes), or the source of exposure (partner and parents), as well as the time of exposure (current or past) or the degree of exposure to SHS.
There is good biological evidence supporting SHS as a risk factor for cancer in never smokers. The chemical composition of SHS is qualitatively similar to mainstream smoke inhaled directly by the smoker, with quantitative differences in carcinogens concentrations and a lower dose of human exposure due to dispersion in the air of the environment (Birkett et al. 2019). Studies based on nicotine and cotinine have suggested relative exposure factors of order 0.06% up to 1% (Pirkle et al. 1996; Office of Population Censuses and Surveys 1996; National Research Council 1986), while for particulate matter of about 0.005–0.26% (Phillips et al. 1996, 1997; National Research Council 1986) and finally for acrolein, a hydrophilic, vapor-phase constituent, the relative dose is estimated to be much higher, 3–31% (National Research Council 1986).
Findings of deoxyribonucleic acid (DNA) adducts in the cervical epithelium as well as nicotine and cotinine in the cervical mucus of SHS-exposed non-smokers support the biological plausibility of the role of SHS in CC risk (CalEPA 2005). Carcinogens in tobacco smoke may cause DNA damage, which then contributes to the development of CC (Ono et al. 2019). Moreover, long-term nicotine exposure may cause physiological effects that raise the likelihood of any cancer, such as increased cell multiplication and suppressed apoptosis (Fonseca-Moutinho 2011). Finally, another theory suggests that smoking may impair women’s antibody response (Simen-Kapeu et al. 2008), affecting both the cellular and humoral immune response leading, respectively, to a persistent infection by inadequate clearance of HPV and to a subsequent infection by inadequate antibody protection (Sopori 2002; Eldridge et al. 2017).
The aim of the present meta-analysis is to provide the most accurate and updated quantification of the association between exposure to SHS and CC risk in non-smoking women giving the functions that best describe the relationships of dose–response between SHS intensity and duration on the risk of CC. We conducted the present meta-analysis using an innovative approach for the identification of original articles (Lugo et al. 2017), and by taking into account the SHS exposure definition and the severity-related CC variables.
Materials and methods
The present study is part of a series of systematic reviews and meta-analyses on the association between cigarette smoking or SHS exposure and cancer risk (Botteri et al. 2020; Liu et al. 2019; Lugo et al. 2018, 2020; Santucci et al. 2019; Scala et al. 2023; Malevolti et al. 2023). It employs an innovative methodology for identifying original articles, which relies on a combination of umbrella and traditional reviews, which has already been described in the previous publications (Lugo et al. 2017, 2018). The study protocol was registered in the International Prospective Registry of Systematic Reviews (PROSPERO; registration number: CRD42017063991).
Search strategy
This meta-analysis was conducted by combining the following two methodologies: umbrella review and traditional review.
As a first step, an umbrella review was held on 10/12/2022, with the main objective of collecting meta-analysis, systematic reviews, and pooled analyses on both active and passive smoking and the risk of cancer at any site. The literature search was run in four databases: Medline, Embase, ISI Wos, and CDSR. In addition, ten out of search reports were added to the results (IARC 2012; IARC 2004; CalEPA 2005; U.S. Department of Health and Human Services 2004; U.S. Department of Health and Human Services 2006; U.S. Department of Health and Human Services 2014; Bonequi et al. 2013; Gankhuyag et al. 2017; Carreras et al. 2019; Shamshirian et al. 2020). Among the 321 eligible meta-analyses, pooled analyses, and systematic reviews, ten were on SHS exposure and CC risk (IARC 2004; CalEPA 2005; U.S. Department of Health and Human Services 2006; IARC 2012; Su et al. 2018; Louie et al. 2011; Kim et al. 2018; Lee et al. 2016; Zeng et al. 2012; Lee 2002). From these 10 studies, we identified 46 non-duplicate articles, which were screened for eligibility on the basis of their full text. According to the eligibility criteria described in the following section, 24 papers were classified as ineligible (Online Supplementary Table 1), resulting in 22 eligible publications from the first step (Online Supplementary Fig. 1).
As a second step, a traditional review was carried out on 10/26/2022 on PubMed/MEDLINE and Embase resulting in a total of 40 articles published from 2018 (the search date of the last and most comprehensive review available on the argument (Su et al. 2018). The search string is reported in Online Supplementary Box 1. After the exclusion of 35 ineligible publications (Online Supplementary Table 1), the update led to five original research articles on the association between SHS exposure and CC risk.
Overall, these two steps of the search strategy resulted in a total of 25 non-duplicate articles being considered as eligible (Online Supplementary Fig. 1).
Eligibility criteria
In the present meta-analysis, we included case–control (including nested case–control or pooled analyses of case–control studies) or cohort studies (including pooled analyses of cohort studies) in English language. Eligible articles should provide information on the association between exposure to SHS and risk of CC in never- or non-smoking (or with a prevalence of ever smokers lower than 5%) women from the general population, thus excluding publications based on women with cancer or other diseases. Cervical cancer includes invasive, i.e., adenocarcinoma, squamous cell carcinoma, adenosquamous cell carcinoma, and CIN, i.e., CIN1, CIN2, CIN3, or carcinoma in situ. Never smokers are women who had never smoked in their lifetime and as non-smokers are given by never and former smokers. Moreover, eligible studies should report risk estimates (including hazard ratios, odds ratios, risk ratios, or mortality rate—all reported as RRs) and corresponding 95% confidence intervals (CIs) for at least one source or setting of SHS exposure or SHS exposure intensity (hour per day, days per week of exposure, and cigarettes per day) or duration (years of exposure) or a combination of them (pack-years of exposure). Also, studies which provide sufficient information to calculate RRs and CIs are eligible.
Data extraction
For each eligible article, we collected information on the publication (e.g., first author, year of publication, and journal), on the study (e.g., country, study name, study period, study design, endpoint, and sample size) and on the CC type. Furthermore, we collected information on the setting of SHS exposure (e.g., workplace, home, and other settings), the source of exposure (e.g., current from partner and during childhood from parents), the model used for RR estimates (including adjusting variables), the RRs with corresponding 95% CIs, and, when available, the number of cases and controls (or at-risk subjects/person-years for cohort studies) for the various exposure categories. In articles where CIs or RR were not reported, they were calculated, if possible. Moreover, we used the Hamling’s methods to analyze the RRs for two or more categories when the reference group was the same (Hamling et al. 2008). Finally, we carefully revised the included studies, to exclude those that were related to studies already included in other articles or that reported the same results as other articles.
Statistical methods
We estimated pooled RRs of CIN of grade 2 or over (CIN2+) and separately for CIN and invasive CC for never and non-smokers exposed to SHS in comparison to non-exposed. We used random-effect meta-analytic models to account for heterogeneity of the risk estimates (DerSimonian and Laird 1986). The χ2 test was used to test for heterogeneity between studies and the I2 statistic to measure proportion of the overall variation attributable to variance between studies (Higgins and Thompson 2002).
We pooled RRs both for each setting (at home—including from partner—at workplace, at home or workplace, in non-specified settings) and source of SHS exposure (from partner, from parents). Moreover we pooled RRs by considering all the types of SHS exposure together and, when a study provided RRs for more than one type of SHS exposure, we selected the RR in accord to the following hierarchy: (1) in non-specified settings, defined as all-sites or non-specified sites of exposure, including clarifications about weekly or daily minimum duration of exposure; (2) at home or at workplace; (3) at home (excluding from partner only); (4) from partner; (5) in non-specified settings, without clarification about weekly or daily minimum duration of exposure; (6) at workplace; (7) during childhood.
We carried out stratified meta-analyses for all types of SHS exposure by study design (i.e., for case–control and cohort studies) and other study characteristics (e.g., geographic area, type of control, year of publication, presence of adjusting variables, and HPV adjustment).
We used funnel plots to assess publication bias (Peters et al. 2008) applying the Egger’s test for funnel plot asymmetry (Egger et al. 1997).
We investigated the relationships between intensity, duration, and pack-years of all types of SHS exposure and the log RR of CC, and we tested linearity through the Wald test (Crippa and Orsini 2016).
All statistical analyses were performed using the R software, version 4.2.2 (‘meta’ and ‘dosresmeta’ packages—Crippa and Orsini 2016).
Results
Characteristics of the selected studies
Out of the 25 identified eligible articles, 4 publications were excluded from the analysis, because they reported duplicated data, leading to 21 original publications included in the present meta-analysis (Online Supplementary Table 1).
The main characteristics of the included studies are reported in Online Supplementary Table 2 (n = 14 case–control studies) and Online Supplementary Table 3 (n = 7 cohort studies). The included articles were published between 1985 and 2022 and were based on more than 5000 CC cases histologically confirmed (1353 CIN, 3879 invasive CC and 4696 CIN2+ cases). Overall, 14 studies provided a measure of the association for SHS exposure at home (including exposure from the partner), 3 for exposure at workplace, 3 for exposure at home or workplace and 8 for exposure in non-specified settings. By considering the sources of SHS exposure, 10 studies provided a measure of the association for SHS from the partner and 4 from the parents or during childhood.
Moreover, 8 studies reported RR estimates for SHS exposure from different settings and sources by intensity (hours per day, days per week, cigarettes per day), 3 by duration (years), and 3 by pack-years.
Meta-analytical results
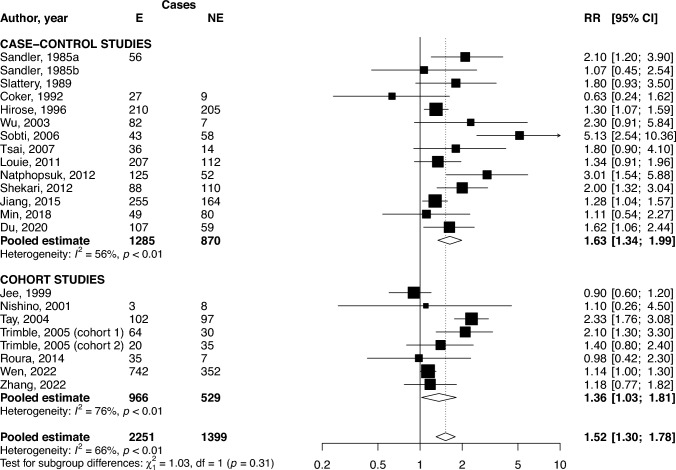
The pooled RR of CIN2+ for women exposed to any type of SHS (i.e., considering one type of exposure for each article) compared to never exposed women was 1.52 (95% CI 1.30–1.78, 21 studies). Stratifying the analysis by type of study, the excess risk of CIN2+ for SHS exposure remains statistically significant in both case–control (RR 1.63, 95% CI 1.34–1.99) and cohort (RR 1.36, 95% CI 1.03–1.81) studies (Fig. 1). Analyses removing those studies that seem outliers (Coker et al. 1992; Sobti et al. 2006; Nishino et al. 2001) were performed. Only Sobti et al. study had an impact on meta-analytical results and heterogeneity: removing it reduces the estimate for CIN2+ to 1.46 (95% CI 1.26–1.68) and to 1.49 (95% CI 1.28–1.74) if restricted to case–control studies, with a reduction in heterogeneity especially in case–control studies (I2 = 59% in overall analysis, I2 = 29% in case–control stratum).
Fig. 1.
Forest plot and pooled relative risk (RR) of cervical intraepithelial neoplasia of grade 2 or higher (CIN2+) for women exposed to second-hand smoke (SHS) compared to non-exposed women, overall and by study design. 95% CI 95% confidence interval, E exposed to SHS, NE non-exposed to SHS
Table 1 shows pooled RRs for the association between all types of SHS exposure and CIN2+ in strata of selected characteristics. Significant differences were observed in the pooled RR for CIN2+ according to the presence of adjustments (RR 2.12, 95% CI 1.59–2.84 in analyses with no adjustment; RR 1.30, 95% CI 1.14–1.48 in analyses with any adjustment). The pooled RR stratified for adjustment by known causes of CC, such as HPV and number of sexual partners, shows higher but non-significantly different RRs in comparison to not adjusted RR. Except for adjusting variables, no stratified analysis showed heterogeneity among strata. Results by setting or source of SHS exposure show a significant increase in the risk of CIN2+ for SHS exposure at home (RR 1.49, 95% CI 1.21–1.84), in non-specified settings (RR 1.64, 95% CI 1.20–2.23) and for SHS exposure from partner (RR 1.55, 95% CI 1.25–1.94). By restricting the analyses to never smokers only (data not shown), the excess risk for CIN2+ in exposed in comparison to non-exposed increases (RR 1.82, 95% CI 1.46–2.27, 11 studies).
Table 1.
Pooled relative risks (RR) and corresponding 95% confidence intervals (CI) for cervical intraepithelial neoplasia of grade 2 or higher (CIN2+) for exposed vs. non-exposed to second-hand smoke (SHS), overall, and in strata of selected characteristics
| Strata | Studies (no.) | Pooled RR for SHS exposure (95% CI) | p value* | p value# |
|---|---|---|---|---|
| Total | 21 | 1.52 (1.30–1.78) | NA | < 0.01 |
| Setting of exposure | ||||
| At homea | 14 | 1.49 (1.21–1.84) | NA | < 0.01 |
| At workplace | 3 | 1.29 (0.79–2.09) | 0.62 | |
| At home or at workplace | 3 | 1.27 (0.83–1.95) | 0.37 | |
| In non-specified settingsb | 8 | 1.64 (1.20–2.23) | < 0.01 | |
| Specific sources | ||||
| From partner | 10 | 1.55 (1.25–1.94) | NA | < 0.01 |
| From parents/during childhood | 4 | 1.25 (0.70–2.24) | 0.08 | |
| Study design | ||||
| Case–control | 14 | 1.63 (1.34–1.99) | 0.31 | < 0.01 |
| Cohortc | 7 | 1.36 (1.03–1.81) | < 0.01 | |
| Type of controlsd | ||||
| Hospital | 9 | 1.72 (1.26–2.35) | 0.20 | < 0.01 |
| Population | 5 | 1.37 (1.16–1.62) | 0.60 | |
| Geographic areae | ||||
| North America | 7 | 1.48 (1.13–1.94) | 0.52 | 0.20 |
| Europe | 1 | 0.98 (0.42–2.29) | NA | |
| Asia | 13 | 1.61 (1.30–1.99) | < 0.01 | |
| Year of publication | ||||
| ≤ 1995 | 11 | 1.42 (1.15–1.75) | 0.30 | < 0.01 |
| ≥ 1996 | 10 | 1.70 (1.30–2.23) | < 0.01 | |
| Any adjustmentf | ||||
| No | 6 | 2.12 (1.59–2.84) | < 0.01 | 0.06 |
| Yes | 15 | 1.30 (1.14–1.48) | 0.08 | |
| HPV adjustment | ||||
| No | 18 | 1.49 (1.27–1.76) | 0.49 | < 0.01 |
| Yes | 3 | 1.84 (1.03–3.29) | 0.14 | |
| Number of sexual partners adjustment | ||||
| No | 17 | 1.49 (1.27–1.76) | 0.69 | < 0.01 |
| Yes | 4 | 1.69 (0.95–2.98) | 0.07 |
aIncluding exposure from partner
bAll-sites or non-specified sites, with or without clarification about weekly or daily minimum duration of exposure
cOne study includes two cohorts (Trimble et al., 2005)
dTypes of control only for case–control studies
eStudies in multiple countries from different areas were not included. There are no data for South America, Oceania, and Africa
fThe most frequent adjustments are age, education level, and number of pregnancies
*p value for heterogeneity across strata. #p value for heterogeneity within strata
The pooled RR of invasive CC for ever exposed to any type of SHS compared to never exposed was 1.42 (95% CI 1.17–1.71, 13 studies; Online Supplementary Fig. 2) and was significantly higher in case–control studies (RR 1.75, 95% CI 1.31–2.32) than in cohort studies (RR 1.11, 95% CI 0.99–1.25). Significant differences were observed in the pooled RR for invasive CC according to the presence of adjustments (p value of heterogeneity across strata = 0.02) with higher risk in studies with no adjustment (Online Supplementary Table 4). By considering the setting or source of SHS exposure, a significant increase in the risk of invasive CC was observed for SHS exposure at home (RR 1.32, 95% CI 1.02–1.69), for SHS exposure in non-specified settings (RR 1.62, 95% CI 1.04–2.54) and for SHS exposure from partner (RR 1.37, 95% CI 1.07–1.77) (Online Supplementary Table 4).
By restricting the analysis to never smokers, only the strength of association does not change (RR for ICC 1.15, 95% CI 1.03–1.27, 6 studies).
For both invasive CC and CIN2+ , no evidence of publication bias emerged either from the visual inspection of the funnel plots (Online Supplementary Figs. 3 and 4, respectively) or from the Egger’s test (p = 0.15 and 0.09 for invasive CC and CIN2+ , respectively).
The pooled RR of CIN for ever exposed to all types of SHS compared to never exposed was 1.50 (95% CI 1.22–1.84; 6 studies; Online Supplementary Fig. 5). Given the small number of studies on CIN, the Egger’s test was not performed and the stratified analyses should be interpreted with caution (Online Supplementary Table 5). By restricting the analysis to never smokers, only the strength of association does not change (RR for CIN 1.32, 95% CI 0.85–2.05, 3 studies).
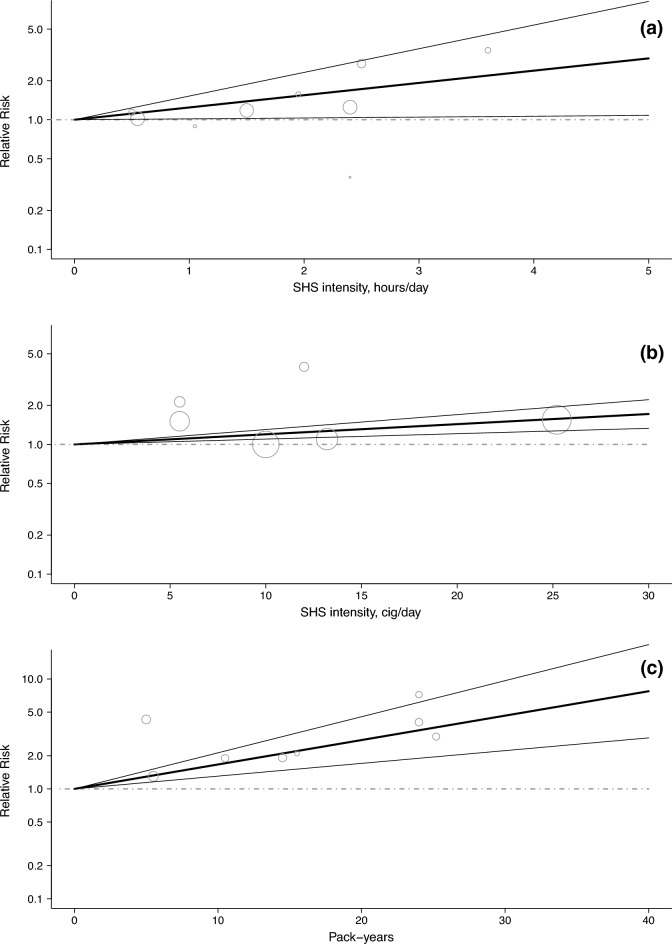
Dose–response analysis
The dose–response analysis was carried out on the risk of CIN2+ only, to pool as many studies as possible. The risk of CIN2+ was found to increase linearly with the intensity of SHS exposure measured as hours of exposure per day from any setting/source (RRs 1.24, 95% CI 1.02–1.52 for 1 h/day; 1.55, 95% CI 1.03–2.32 for 2 h/day and 2.39, 95% CI 1.06–5.37 for 4 h/day; based on 4 studies; Fig. 2a). A similar trend was observed for intensity measured as the number of cigarettes of SHS exposure per day (RRs 1.20, 95% CI 1.10–1.30 for 10 cigarettes/day and 1.43, 95% CI 1.21–1.70 for 20 cigarettes/day; based on 3 studies; Fig. 2b). The risk also increased linearly with pack-years of exposure (RR 1.67, 95% CI 1.31–2.13 for 10 pack-years, 2.78, 95% CI 1.71–4.53 for 20 pack-years; Fig. 2c).
Fig. 2.
Relative risk (RR) for the dose–response relationships between second-hand smoke (SHS) exposure intensity and pack-years, and the risk of cervical intraepithelial neoplasia of grade 2 or higher (CIN2+). a Hours per day of SHS exposure (based on four studies). b Cigarettes per day of SHS exposure (based on three studies). c Pack-years of SHS exposure (based on three studies). Bold line: linear regression model; line: 95% confidence interval of the restricted cubic spline or linear model; dotted line: RR for the reference category (non-exposed); bubble: RR for various exposure categories in each study included in the analysis, where the area of the circle is proportional to the precision of the RR (i.e., to the inverse variance)
The relationship between duration of SHS exposure and risk of CC was based on very few and heterogeneous studies resulting in an uncertain and unclear trend (data not shown). The estimated functions to model the dose–response curves are reported in Online Supplementary Box 2.
Discussion
In this systematic review and meta-analysis, we comprehensively summarized the most up-to-date evidence from 21 original articles on the association between SHS exposure and the risk of CC among female non-smokers. Consistently with a previous meta-analysis (Su et al. 2018), women exposed to any type of SHS showed a significant 52% increase in the risk of CIN2+ compared to unexposed women. Similar results were found for invasive CC and CIN.
By analyzing the different settings and sources of SHS exposure, the risk of CC resulted significantly higher for women exposed at home, in non-specified settings and from the partner compared to non-exposed women, whereas a non-significant RR for SHS exposure at workplace or from parents was found. These results suggest that household (comprising partner) exposure may play a large role in the development of CC related to SHS exposure, whereas past exposure (i.e., from parents or during childhood) has a low impact in the development of CC. However, stratified meta-analyses should be interpreted with caution given the small number of involved studies.
We found the RR from cohort studies to be lower than those from case–control studies for CIN2+ and invasive CC. However, it is important to highlight that, regardless of the study design, an overall direct association between SHS exposure and CC risk was found.
Our results showed a substantial heterogeneity, especially for the risk of CIN2+ and invasive CC, which, however, was reduced by becoming influential by restricting the analyses to specific strata.
We performed a stratified analysis by year of publication (before 1995 and after 1996) with the aim of taking into account the spread of screening programs, which occurred on average around 1996. This analysis did not show differences in CC risks, highlighting that preventive programs have no role in the association between SHS and CC.
The pooled RR for CC resulted higher in studies without adjustment (significantly different for CIN2+ and invasive CC), suggesting that part of the association between SHS exposure and CC risk is due to adjustment variables. The excess risk of CIN2+ , invasive CC and CIN in studies with adjustment remains however, respectively, 30%, 21%, and 41% higher in women exposed to SHS in comparison to unexposed ones. Adjustment in the included studies is likely to be complete, since over 80% of studies adjust for more than three variables, mainly age, pregnancy, and education level (Online Supplementary Tables 2 and 3). Specific stratified analyses for the well-known causes of CC, such as HPV infection and number of sexual partners, suggest that, net of such risk factors, the RR for CC in higher in SHS exposed in comparison to not exposed, but there are other adjusting variables that capture part of the association between SHS and CC.
Additionally, our study found linear dose–response relationships for both intensity and pack-years of SHS exposure and the risk of CIN2+ . As an example, the risk of CIN2+ resulted more than doubled in women with 4 h/day of exposure, compared to unexposed women. However, these findings are based on a limited number of studies and should be therefore confirmed by future studies.
A recent meta-analysis reported a CIN2+ RR of 1.99 in current and 1.29 in former smokers in comparison to never smokers (Malevolti et al. 2023), suggesting that active and passive smoking could be considered among the most important modifiable risk factors for the development of CC. Given the lower association of former smoking with CC in comparison to current and passive smoking, the reduction of smoking and consequently of exposure to SHS could be the more effective strategy to reduce the incidence of CC at the population level.
Our meta-analysis has several strengths. First of all, the innovative methodology combining an umbrella and a traditional review allowed us to include a large number of studies on the issue (Lugo et al. 2017). The traditional review is carried out as an update of the last more comprehensive review on the topic, whose quality could, however, impact the results. Second, duplicate data were excluded after a careful articles’ revision. Additionally, we examined different settings and sources of SHS exposure, allowing for a more precise and comprehensive understanding of the relationship between SHS and CC. Finally, we investigated dose–response relationships, to better explain the observed associations.
This meta-analysis has some limitations. The first is presence of heterogeneity between studies that was, however, taken into account using random-effects models and conducting a number of stratified analyses to identify and reduce possible sources of heterogeneity. Another limitation is due to the fact that several stratified analyses as well as the dose–response models are based on a small number of studies and results should therefore be interpreted with caution. In addition, another limitation common to all meta-analyses is due to the fact that case–control and cohort studies are susceptible to recall and selection biases. In particular, information on cigarette smoking was self-reported in all included studies, and therefore, it could be misclassified, also because current or former smokers are known to deny having smoked (Lee and Forey 1995; Connor-Gorber et al. 2009). The bias due to misclassification of smokers could be higher in analysis on SHS exposure from partner, due to the fact that smokers tend to marry smokers (Lee 1992; Hackshaw et al. 1997). Moreover, as in the previous systematic reviews that we conducted on the association between smoking or SHS exposure and cancer at various sites (Botteri et al. 2020; Liu et al. 2019; Lugo et al. 2018, 2020; Santucci et al. 2019; Scala et al. 2023; Malevolti et al. 2023), we decided not to perform a quality assessment of eligible studies. The recommended tool for assessing the quality of observational studies is the Newcastle–Ottawa Quality Assessment Scale (Zeng et al. 2015) which evaluates the quality of reporting results rather than the methodology itself and it can assign a low quality to large studies whose methodology is reported in protocols or other publications. Furthermore, rating of some items is subjective. We preferred to conduct stratified analyses based on some variables that may concur in the quality of observational studies, e.g., type of controls, exposure definition, or adjustment.
Conclusions
In conclusion, results from this comprehensive and up-to date review and meta-analysis suggest that exposure to SHS significantly increases the risk of developing CC. Despite the lower dose of carcinogens contained in passive smoking compared to that in active, the RRs for SHS are a large part of that for active smoking, and further studies are needed to investigate this aspect. These results should make women and the population aware of the risk associated with exposure to SHS, still present, especially in private settings.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The research leading to these results has received funding from AIRC (Associazione italiana per la Ricerca sul Cancro) under MFAG (My First AIRC Grant) 2021—ID. 25840 project—P.I. Lugo Alessandra. The work of SG is partially supported by the Italian League Against Cancer (LILT, Milan). The work of GC and MCM is partially supported by the 2018 Health Research Grant by the Tuscany Region within the project ‘Attributable CAncer Burden in Tuscany: smoking, environmental and occupational risk factors, and evaluation of prevention strategies’ (ACAB).
Author contributions
All authors contributed to the study conception and design. Material preparation was performed by GC and AL. Data collection was performed by MCM and CM. Analysis was performed by MCM. The first draft of the manuscript was written by GC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by AIRC under MFAG 2021—ID. 25840 project—P.I. Lugo Alessandra, Italian League Against Cancer (LILT, Milan) and Regione Toscana Bando Ricerca Salute 2018 (Project: Attributable CAncer Burden in Tuscany: smoking, environmental and occupational risk factors and evaluation of prevention strategies, ACAB)—P.I. Giulia Carreras.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval
This is an observational study on published aggregated measures. No ethical approval is required.
Consent to participate
This is an observational study on published aggregated measures. Informed consent is not applicable.
Consent to publish
This is an observational study on published aggregated measures. Consent to publish is not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Birkett N, Al-Zoughool M, Bird M et al (2019) Overview of biological mechanisms of human carcinogens. J Toxicol Environ Health B Crit Rev 22(7–8):288–359. 10.1080/10937404.2019.1643539 [DOI] [PubMed] [Google Scholar]
- Bonequi P, Meneses-González F, Correa P et al (2013) Risk factors for gastric cancer in Latin America: a meta-analysis. Cancer Causes Control 24(2):217–231. 10.1007/s10552-012-0110-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botteri E, Borroni E, Sloan EK et al (2020) Smoking and colorectal cancer risk, overall and by molecular subtypes: a meta-analysis. Am J Gastroenterol 115(12):1940–1949. 10.14309/ajg.0000000000000803 [DOI] [PubMed] [Google Scholar]
- California Environmental Protection Agency (2005) Proposed identification of environmental tobacco smoke as a toxic air contaminant. Sacramento. https://escholarship.org/uc/item/8hk6960q
- Carreras G, Lugo A, Gallus S et al (2019) Burden of disease attributable to second-hand smoke exposure: a systematic review. Prev Med 129:105833. 10.1016/j.ypmed.2019.105833 [DOI] [PubMed] [Google Scholar]
- Castellsagué X, Muñoz N (2003) Chapter 3: cofactors in human papillomavirus carcinogenesis–role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr 2003(31):20–28 [PubMed] [Google Scholar]
- Coker AL, Rosenberg AJ, McCann MF et al (1992) Active and passive cigarette smoke exposure and cervical intraepithelial neoplasia. Cancer Epidemiol Biomark Prev 1(5):349–356 [PubMed] [Google Scholar]
- Connor-Gorber S, Schofield-Hurwitz S, Hardt J et al (2009) The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res 11(1):12–24 [DOI] [PubMed] [Google Scholar]
- Crippa A, Orsini N (2016) Multivariate dose-response meta-analysis: the dosresmeta R package. J Stat Softw 72(1):1–15. 10.18637/jss.v072.c01 [Google Scholar]
- DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M et al (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge RC, Pawlita M, Wilson L et al (2017) Smoking and subsequent human papillomavirus infection: a mediation analysis. Ann Epidemiol 27(11):724-730.e1. 10.1016/j.annepidem.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca-Moutinho JA (2011) Smoking and cervical cancer. ISRN Obstet Gynecol 2011(847684):1–6. 10.5402/2011/847684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gankhuyag N, Lee KH, Cho JY (2017) The role of nitrosamine (NNK) in breast cancer carcinogenesis. J Mammary Gland Biol Neoplasia 22(3):159–170. 10.1007/s10911-017-9381-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackshaw AK, Law MR, Wald NJ (1997) The accumulated evidence on lung cancer and environmental tobacco smoke. BMJ 315:980–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamling J, Lee P, Weitkunat R et al (2008) Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 27(7):954–970. 10.1002/sim.3013 [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- Hirose K, Tajima K, Hamajima N et al (1996) Subsite (cervix/endometrium)-specific risk and protective factors in uterus cancer. Jpn J Cancer Res 87(9):1001–1009. 10.1111/j.1349-7006.1996.tb02132.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research and Cancer (2004) Tobacco smoke and involuntary smoking. In: IARC Monographs on the evaluation of carcinogenic risks to humans, vol 83. IARC, Lyon [PMC free article] [PubMed]
- International Agency for Research and Cancer (2012) A review of human carcinogens: personal habits and indoor combustions. In: IARC monographs on the evaluation of carcinogenic risks to humans. IARC, Lyon [PMC free article] [PubMed]
- Kim AS, Ko HJ, Kwon JH et al (2018) Exposure to secondhand smoke and risk of cancer in never smokers: a meta-analysis of epidemiologic studies. Int J Environ Res Public Health 15(9):1981. 10.3390/ijerph15091981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiol J, Lindsay L, Pimenta JM et al (2008) Persistent human papillomavirus infection and cervical neoplasia: a systematic review and meta-analysis. Am J Epidemiol 168(2):123–137. 10.1093/aje/kwn036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PN (1992) Environmental tobacco smoke and mortality. A detailed review of epidemiological evidence relating environmental tobacco smoke to the risk of cancer, heart disease and other causes of death in adults who have never smoked. Karger
- Lee PN (2002) Environmental tobacco smoke and cancer of sites other than the lung in adult non-smokers. Food Chem Toxicol 40(6):747–766. 10.1016/S0278-6915(02)00027-3 [DOI] [PubMed] [Google Scholar]
- Lee PN, Forey BA (1995) Misclassification of smoking habits as determined by cotinine or by repeated self-report—a summary of evidence from 42 studies. J Smoking-Related Dis 6:109–129 [Google Scholar]
- Lee PN, Thornton AJ, Hamling JS (2016) Epidemiological evidence on environmental tobacco smoke and cancers other than lung or breast. Regul Toxicol Pharmacol 80:134–163. 10.1016/j.yrtph.2016.06.012 [DOI] [PubMed] [Google Scholar]
- Liu X, Peveri G, Bosetti C et al (2019) Dose-response relationships between cigarette smoking and kidney cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol 142:86–93. 10.1016/j.critrevonc.2019.07.019 [DOI] [PubMed] [Google Scholar]
- Louie KS, Castellsague X, de Sanjose S et al (2011) Smoking and passive smoking in cervical cancer risk: pooled analysis of couples from the IARC multicentric case-control studies. Cancer Epidemiol Biomarkers Prev 20(7):1379–1390. 10.1158/1055-9965.EPI-11-0284 [DOI] [PubMed] [Google Scholar]
- Lugo A, Bosetti C, Peveri G et al (2017) Dose-response relationship between cigarette smoking and site-specific cancer risk: protocol for a systematic review with an original design combining umbrella and traditional reviews. BMJ Open 7(10):e018930. 10.1136/bmjopen-2017-018930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo A, Peveri G, Bosetti C et al (2018) Strong excess risk of pancreatic cancer for low frequency and duration of cigarette smoking: a comprehensive review and meta-analysis. Eur J Cancer 104:117–126. 10.1016/j.ejca.2018.09.007 [DOI] [PubMed] [Google Scholar]
- Lugo A, Peveri G, Gallus S (2020) Should we consider gallbladder cancer a new smoking-related cancer? A comprehensive meta-analysis focused on dose–response relationships. Int J Cancer 146(12):3304–3311. 10.1002/ijc.32681 [DOI] [PubMed] [Google Scholar]
- Malevolti MC, Lugo A, Scala M et al (2023) Dose-risk relationships between cigarette smoking and cervical cancer: a systematic review and meta-analysis. Eur J Cancer Prev 32(2):171–183. 10.1097/CEJ.0000000000000773 [DOI] [PubMed] [Google Scholar]
- Min KJ, Lee JK, So KA et al (2018) Association between passive smoking and the risk of cervical intraepithelial neoplasia 1 in Korean women. J Epidemiol 28(1):48–53. 10.2188/jea.JE20160118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (US) (1986) Committee on Passive Smoking. Environmental tobacco smoke. Measuring Exposures and Assessing Health Effects. Washington (DC): National Academies Press (US) [PubMed]
- Natphopsuk S, Settheetham-Ishida W, Sinawat S et al (2012) Risk factors for cervical cancer in Northeastern Thailand: detailed analyses of sexual and smoking behavior. Asian Pac J Cancer Prev 13(11):5489–5495. 10.7314/APJCP.2012.13.11.5489 [DOI] [PubMed] [Google Scholar]
- Nishino Y, Tsubono Y, Tsuji I et al (2001) Passive smoking at home and cancer risk: a population-based prospective study in Japanese nonsmoking women. Cancer Causes Control 12(9):797–802. 10.1023/a:1012273806199 [DOI] [PubMed] [Google Scholar]
- Office of Population Censuses and Surveys (1996) Health survey for England 1994. Volume I: Findings. Volume II: Survey methodology & documentation. Colhoun H, Prescott-Clarke P eds. HMSO, London
- Ono A, Nakagawa M, Ikuta E et al (2019) Relationship between tobacco smoking and cervical cancer. Womens Health Open J 5(1):19–21. 10.17140/WHOJ-5-133 [Google Scholar]
- Peters JL, Sutton AJ, Jones DR et al (2008) Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 61(10):991–996. 10.1016/j.jclinepi.2007.11.010 [DOI] [PubMed] [Google Scholar]
- Phillips K, Bentley MC, Howard DA et al (1996) Assessment of air quality in Stockholm by personal monitoring of nonsmokers for respirable suspended particles and environmental tobacco smoke. Scand J Work Environ Health 22(Suppl):1 [PubMed] [Google Scholar]
- Phillips K, Howard DA, Bentley MC et al (1997) Assessment of air quality in Turin by personal monitoring of nonsmokers for respirable suspended particles and environmental tobacco smoke. Environ Int 23:851–871. 10.1016/S0160-4120(97)00097-4 [PubMed] [Google Scholar]
- Pirkle JL, Flegal KM, Bernert JT et al (1996) Exposure of the US population to environmental tobacco smoke. The Third National Health and Nutrition Examination Survey, 1988 to 1991. JAMA 275:1233–1240. 10.1001/jama.1996.03530400021033 [PubMed] [Google Scholar]
- Santucci C, Bosetti C, Peveri G et al (2019) Dose–risk relationships between cigarette smoking and ovarian cancer histotypes: a comprehensive meta-analysis. Cancer Causes Control 30(9):1023–1032. 10.1007/s10552-019-01198-8 [DOI] [PubMed] [Google Scholar]
- Scala M, Bosetti C, Bagnardi V et al (2023) Dose-response relationships between cigarette smoking and breast cancer risk: a systematic review and meta-analysis. J Epidemiol. 10.2188/jea.JE20220206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamshirian A, Heydari K, Shams Z et al (2020) Breast cancer risk factors in Iran: a systematic review & meta-analysis. Horm Mol Biol Clin Investig. 10.1016/j.ypmed.2019.105833 [DOI] [PubMed] [Google Scholar]
- Simen-Kapeu A, Kataja V, Yliskoski M et al (2008) Smoking impairs human papillomavirus (HPV) type 16 and 18 capsids antibody response following natural HPV infection. Scand J Infect Dis 40(9):745–751. 10.1080/00365540801995360 [DOI] [PubMed] [Google Scholar]
- Slattery ML, Robison LM, Schuman KL et al (1989) Cigarette smoking and exposure to passive smoke are risk factors for cervical cancer. JAMA 261(11):1593–1598. 10.1001/jama.1989.03420110069026 [PubMed] [Google Scholar]
- Sobti RC, Kaur S, Kaur P et al (2006) Interaction of passive smoking with GST (GSTM1, GSTT1, and GSTP1) genotypes in the risk of cervical cancer in India. Cancer Genet Cytogenet 166(2):117–123. 10.1016/j.cancergencyto.2005.10.001 [DOI] [PubMed] [Google Scholar]
- Sopori M (2002) Effects of cigarette smoke on the immune system. Nat Rev Immunol 2(5):372–377. 10.1038/nri803 [DOI] [PubMed] [Google Scholar]
- Su B, Qin W, Xue F et al (2018) The relation of passive smoking with cervical cancer: a systematic review and meta-analysis. Medicine (baltimore) 97(46):e13061. 10.1097/MD.0000000000013061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- Trimble CL, Genkinger JM, Burke AE, Hoffman SC, Helzlsouer KJ, Diener-West M et al (2005) Active and Passive Cigarette Smoking and the Risk of Cervical Neoplasia. Obstet Gynecol Gennaio 105(1):174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (2004) The health consequences of smoking. A report of the Surgeon General, Atlanta
- U.S. Department of Health and Human Services (2006) The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General, Atlanta [PubMed]
- U.S. Department of Health and Human Services (2014) The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General, Atlanta
- Walboomers JMM, Jacobs MV, Manos MM et al (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189(1):12–19. 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- Wen Q, Wang X, Lv J et al (2022) Association between involuntary smoking and risk of cervical cancer in Chinese female never smokers: a prospective cohort study. Environ Res 212(Pt C):113371. 10.1016/j.envres.2022.113371 [DOI] [PubMed] [Google Scholar]
- Wu MT, Lee LH, Ho CK et al (2003) Lifetime exposure to environmental tobacco smoke and cervical intraepithelial neoplasms among nonsmoking Taiwanese women. Arch Environ Health 58(6):353–359 [PubMed] [Google Scholar]
- Zeng XT, Xiong PA, Wang F et al (2012) Passive smoking and cervical cancer risk: a meta-analysis based on 3,230 cases and 2,982 controls. Asian Pac J Cancer Prev 13(6):2687–2693. 10.7314/APJCP.2012.13.6.2687 [DOI] [PubMed] [Google Scholar]
- Zeng X, Zhang Y, Kwong JS et al (2015) The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 8:2–10. 10.1111/jebm.12141 [DOI] [PubMed] [Google Scholar]
- Zhang S, Xu H, Zhang L et al (2020) Cervical cancer: epidemiology, risk factors and screening. Chin J Cancer Res 32(6):720–728. 10.21147/j.issn.1000-9604.2020.06.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Li Z, Zhang X et al (2022) Association between secondhand smoke and cancers in adults in the US population. J Cancer Res Clin Oncol. 10.1007/s00432-022-04266-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.