Abstract
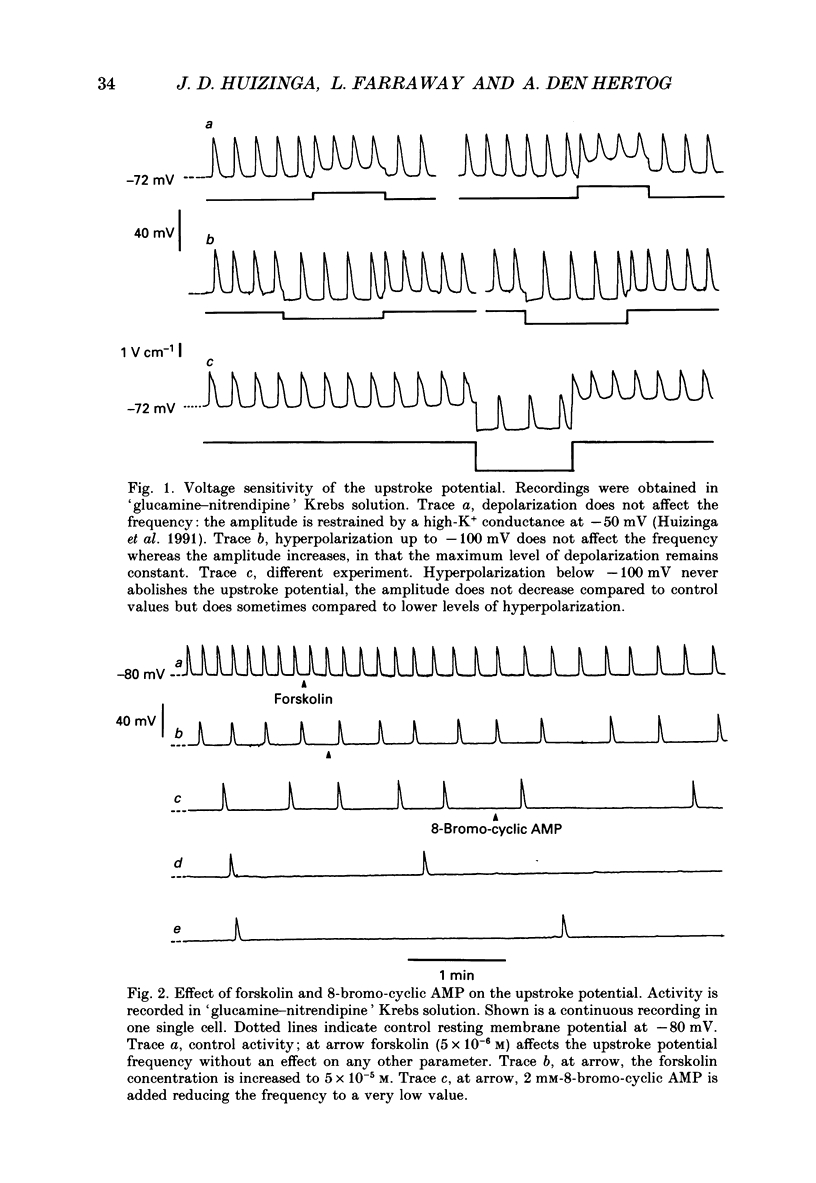
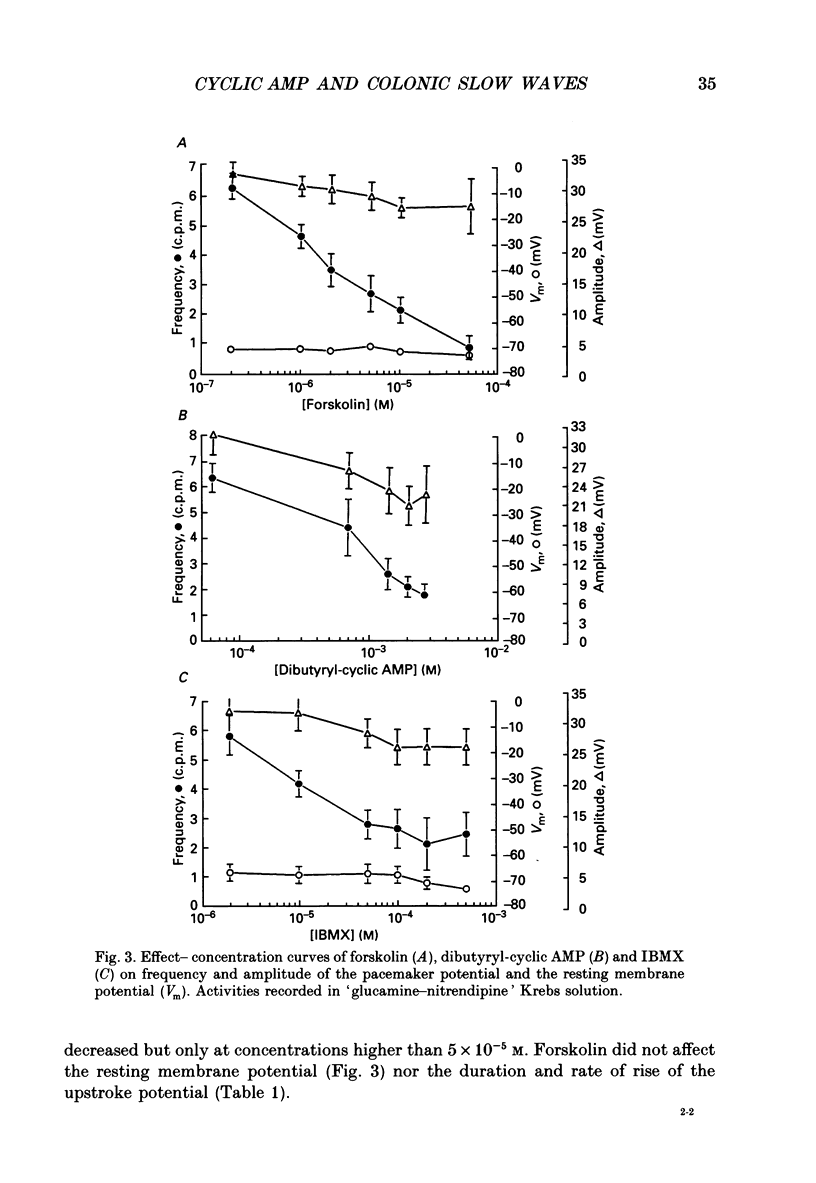
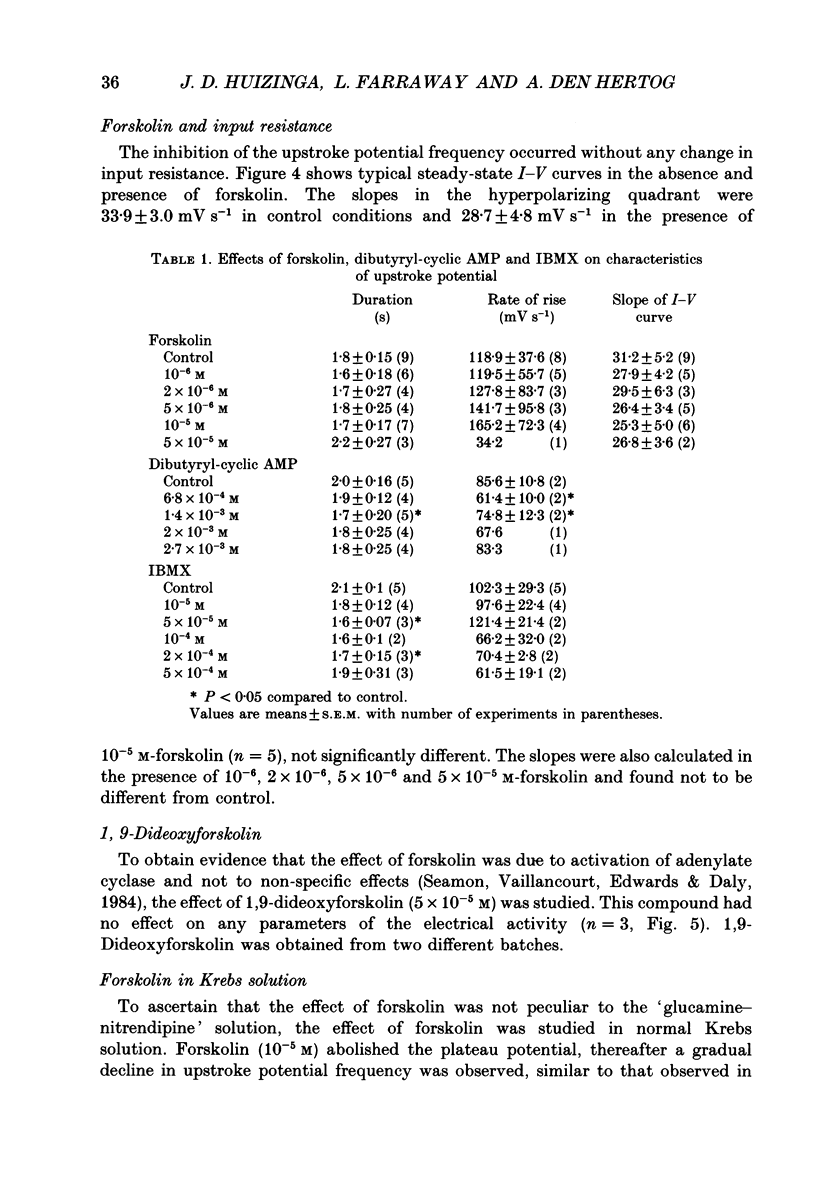
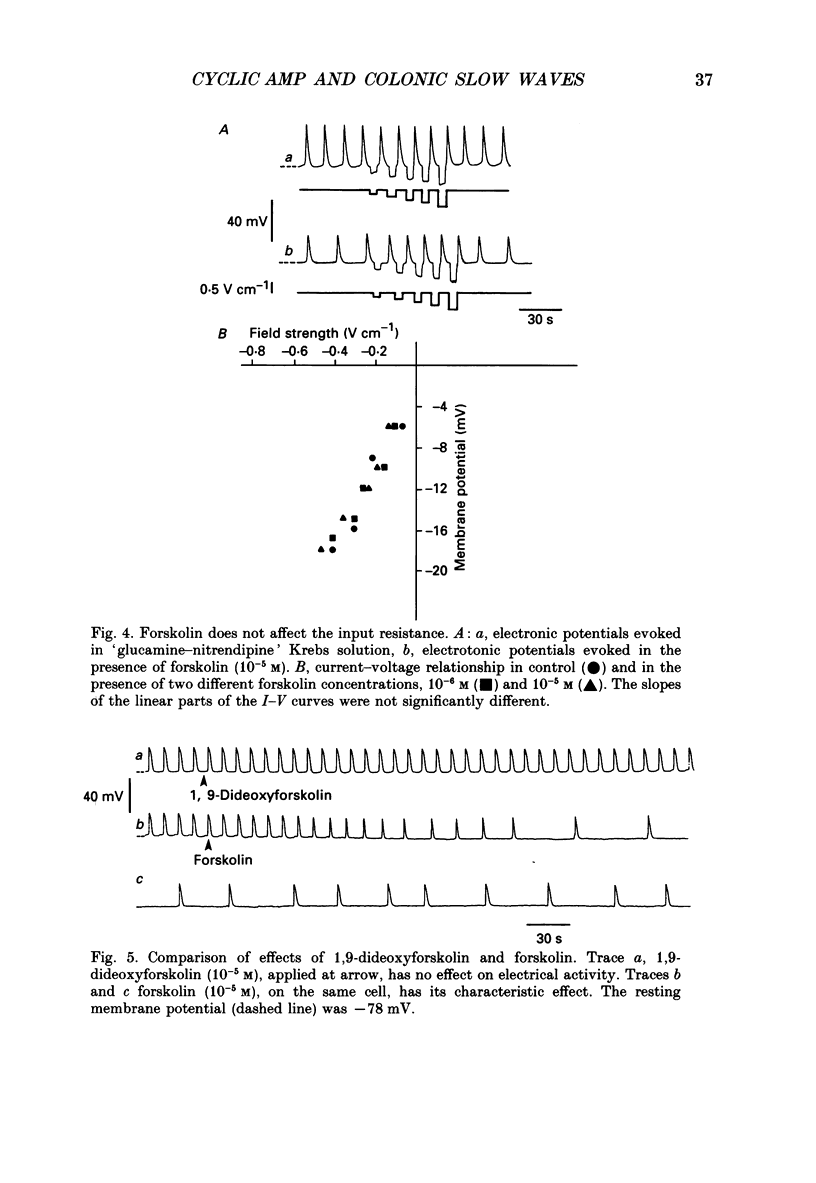
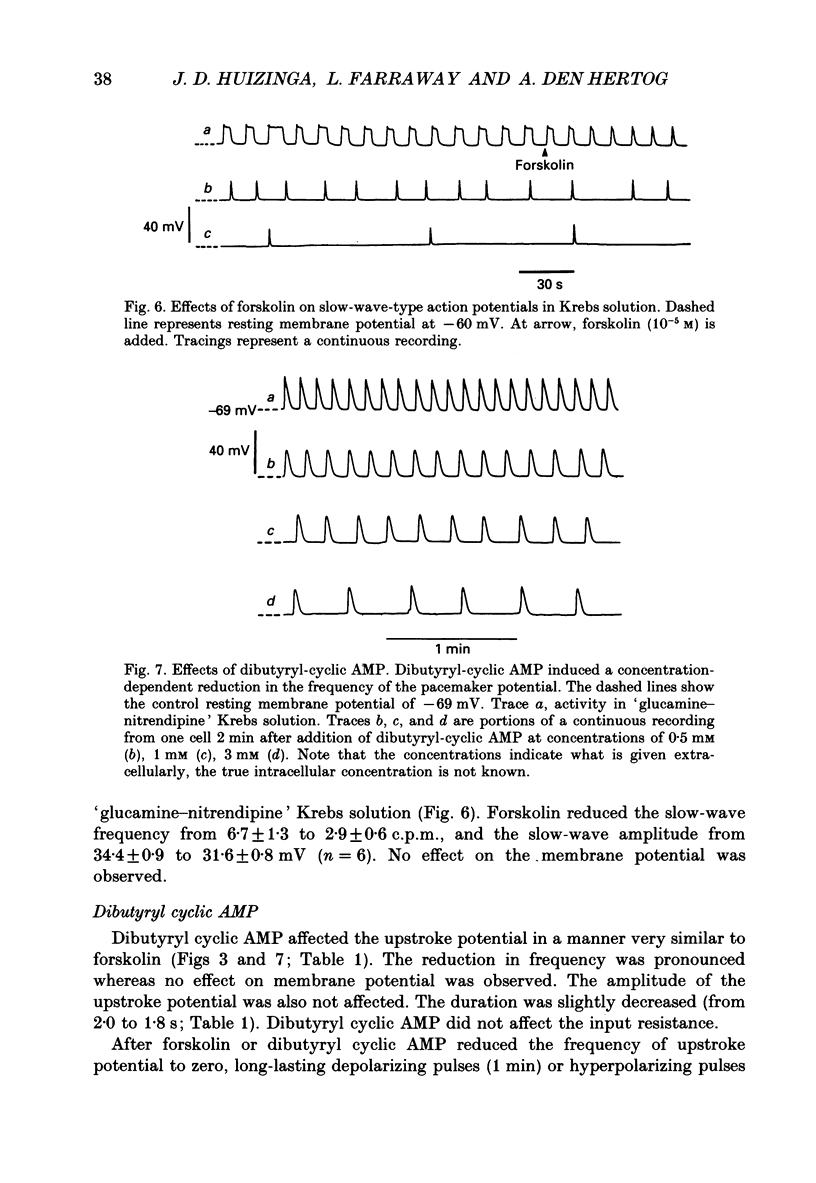
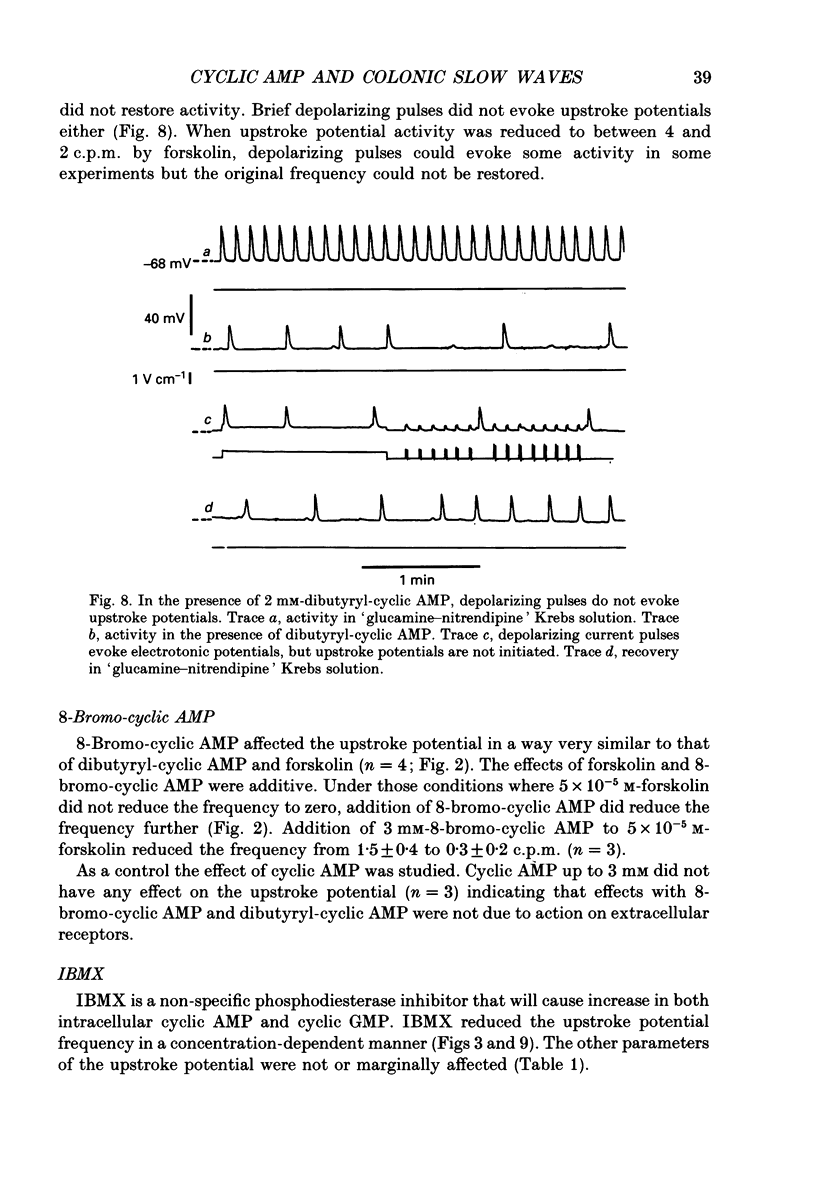
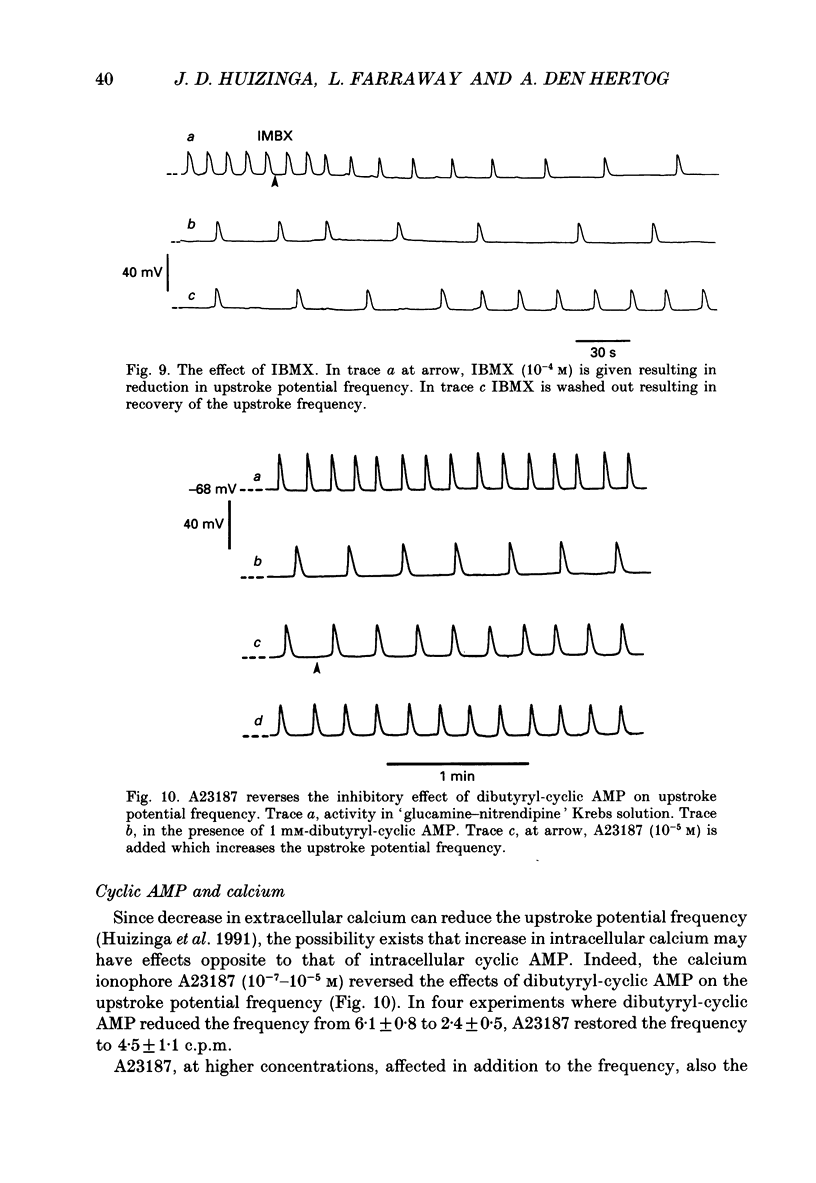
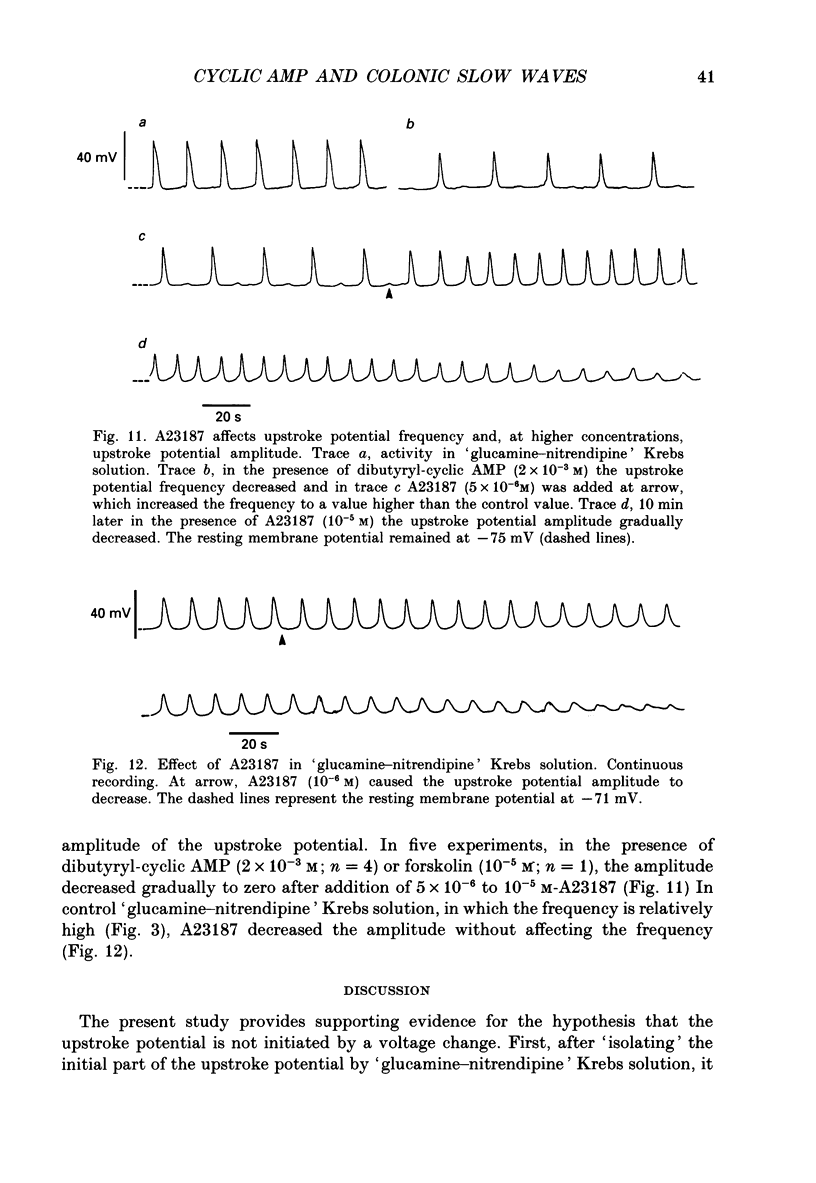
1. A non-L-type calcium conductance is involved in the generation of the initial part of the slow-wave-type action potential in colonic smooth muscle. The present study addresses the question whether this conductance is voltage or metabolically activated. 2. Current-induced hyperpolarization increased frequency and amplitude of slow waves measured in Krebs solution. 3. The upstroke potential was 'isolated' from the slow wave by superfusion with 'glucamine-nitrendipine' Krebs solution (NaCl was replaced by glucamine, nitrendipine was added). 4. Hyperpolarization up to -100 mV did not affect the upstroke potential frequency and increased its amplitude. Only hyperpolarization further than -100 mV decreased the frequency less than or equal to 20%, and reduced the amplitude less than or equal to 20%. 5. Depolarization did not affect the upstroke potential frequency. 6. Forskolin, but not 1,9-dideoxyforskolin dramatically decreased the upstroke potential frequency, without affecting other parameters including the resting membrane potential. 7. The effect of forskolin was mimicked by dibutyryl cyclic AMP, 8-bromo-cyclic AMP and 3-isobutyl-1-methylxanthine (IBMX), but not extracellular cyclic AMP. 8. The upstroke potential could not be evoked by depolarizing pulses after inhibition of activity by forskolin. 9. The effect of forskolin could be reversed by the calcium ionophore A23187. 10. In summary, voltage changes up to -40 mV and down to -100 mV do not, but changes in intracellular cyclic AMP do affect the frequency of the upstroke potential. 11. It is likely that intracellular metabolic activity, which may include cyclic AMP but not a voltage change, activates the conductance responsible for the generation of the upstroke potential.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barajas-López C., Berezin I., Daniel E. E., Huizinga J. D. Pacemaker activity recorded in interstitial cells of Cajal of the gastrointestinal tract. Am J Physiol. 1989 Oct;257(4 Pt 1):C830–C835. doi: 10.1152/ajpcell.1989.257.4.C830. [DOI] [PubMed] [Google Scholar]
- Barajas-López C., Chow E., Den Hertog A., Huizinga J. D. Role of the sodium pump in pacemaker generation in dog colonic smooth muscle. J Physiol. 1989 Sep;416:369–383. doi: 10.1113/jphysiol.1989.sp017766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barajas-López C., Den Hertog A., Huizinga J. D. Ionic basis of pacemaker generation in dog colonic smooth muscle. J Physiol. 1989 Sep;416:385–402. doi: 10.1113/jphysiol.1989.sp017767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barajas-López C., Huizinga J. D. Heterogeneity in spontaneous and tetraethylammonium induced intracellular electrical activity in colonic circular muscle. Pflugers Arch. 1988 Jul;412(1-2):203–210. doi: 10.1007/BF00583751. [DOI] [PubMed] [Google Scholar]
- Bardakjian B. L., Lau M. Y. The refractory properties of mapped clock oscillators representing smooth muscle electrical oscillations. Prog Clin Biol Res. 1990;327:627–634. [PubMed] [Google Scholar]
- Bauer A. J., Sanders K. M. Gradient in excitation-contraction coupling in canine gastric antral circular muscle. J Physiol. 1985 Dec;369:283–294. doi: 10.1113/jphysiol.1985.sp015901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezin I., Huizinga J. D., Daniel E. E. Interstitial cells of Cajal in the canine colon: a special communication network at the inner border of the circular muscle. J Comp Neurol. 1988 Jul 1;273(1):42–51. doi: 10.1002/cne.902730105. [DOI] [PubMed] [Google Scholar]
- Chow E., Huizinga J. D. Myogenic electrical control activity in longitudinal muscle of human and dog colon. J Physiol. 1987 Nov;392:21–34. doi: 10.1113/jphysiol.1987.sp016767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A. On exploring the basis for slow potential oscillations in the mammalian stomach and intestine. J Exp Biol. 1979 Aug;81:153–173. doi: 10.1242/jeb.81.1.153. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Prosser C. L., Weems W. A. A study of pace-maker activity in intestinal smooth muscle. J Physiol. 1974 Aug;240(3):671–701. doi: 10.1113/jphysiol.1974.sp010629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahms V., Prosser C. L., Suzuki N. Two types of 'slow waves' in intestinal smooth muscle of cat. J Physiol. 1987 Nov;392:51–69. doi: 10.1113/jphysiol.1987.sp016769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkawy T. Y., Daniel E. E. Electrical activity of small intestinal smooth muscle and its temperature dependence. Am J Physiol. 1975 Nov;229(5):1268–1276. doi: 10.1152/ajplegacy.1975.229.5.1268. [DOI] [PubMed] [Google Scholar]
- Hara Y., Kubota M., Szurszewski J. H. Electrophysiology of smooth muscle of the small intestine of some mammals. J Physiol. 1986 Mar;372:501–520. doi: 10.1113/jphysiol.1986.sp016022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga J. D., Chow E. Electrotonic current spread in colonic smooth muscle. Am J Physiol. 1988 May;254(5 Pt 1):G702–G710. doi: 10.1152/ajpgi.1988.254.5.G702. [DOI] [PubMed] [Google Scholar]
- Huizinga J. D., Farraway L., Den Hertog A. Generation of slow-wave-type action potentials in canine colon smooth muscle involves a non-L-type Ca2+ conductance. J Physiol. 1991 Oct;442:15–29. doi: 10.1113/jphysiol.1991.sp018779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga J. D., Walton P. D. Pacemaker activity in the proximal lower oesophageal sphincter of the dog. J Physiol. 1989 Jan;408:19–30. doi: 10.1113/jphysiol.1989.sp017443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magaribuchi T., Obu T., Sakamoto Y., Yamamoto Y. Some electrical properties of the slow potential changes recorded from the guinea pig stomach in relation to drug actions. Jpn J Physiol. 1972 Jun;22(3):333–352. doi: 10.2170/jjphysiol.22.333. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Vaillancourt R., Edwards M., Daly J. W. Binding of [3H]forskolin to rat brain membranes. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5081–5085. doi: 10.1073/pnas.81.16.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum S. A., Camardo J. S., Kandel E. R. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982 Sep 30;299(5882):413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- Smith T. K., Reed J. B., Sanders K. M. Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. Am J Physiol. 1987 Feb;252(2 Pt 1):C215–C224. doi: 10.1152/ajpcell.1987.252.2.C215. [DOI] [PubMed] [Google Scholar]
- Sperelakis N. Regulation of calcium slow channels of cardiac muscle by cyclic nucleotides and phosphorylation. J Mol Cell Cardiol. 1988 Mar;20 (Suppl 2):75–105. doi: 10.1016/0022-2828(88)90334-3. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Prosser C. L., Dahms V. Boundary cells between longitudinal and circular layers: essential for electrical slow waves in cat intestine. Am J Physiol. 1986 Mar;250(3 Pt 1):G287–G294. doi: 10.1152/ajpgi.1986.250.3.G287. [DOI] [PubMed] [Google Scholar]


