Abstract
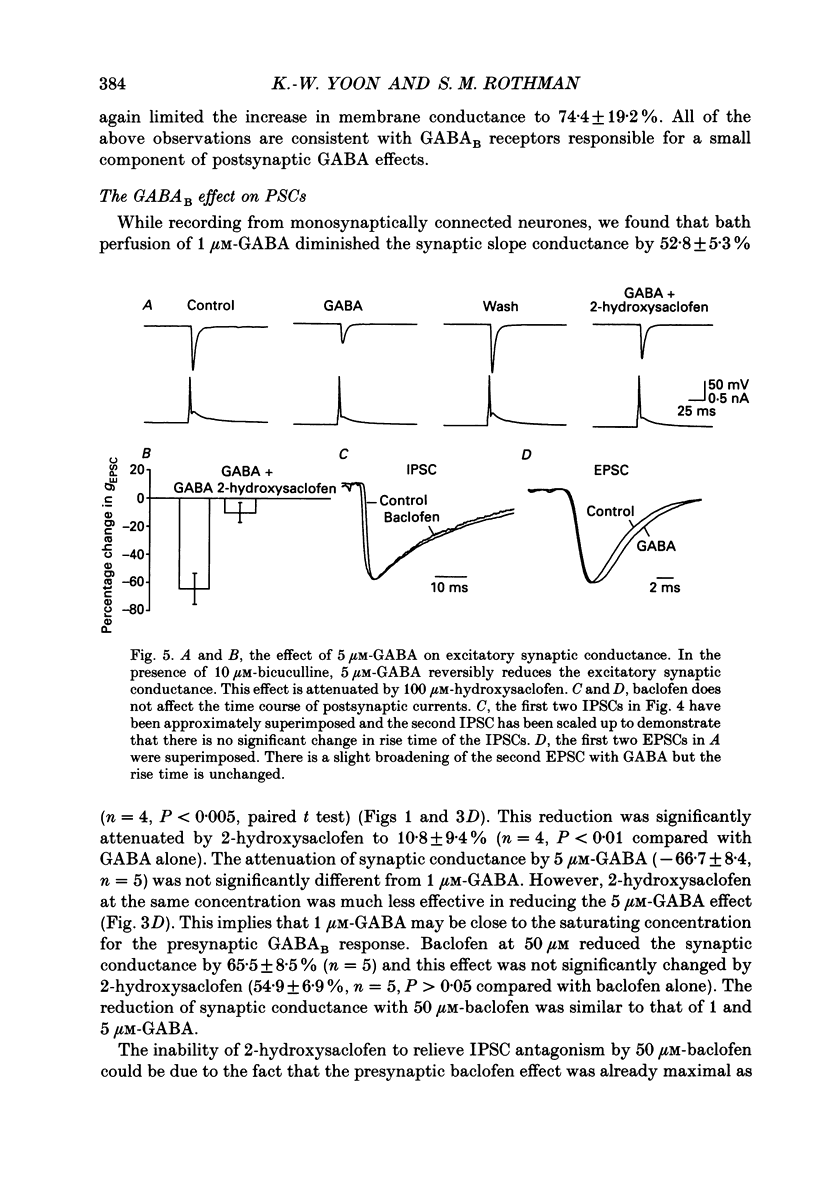
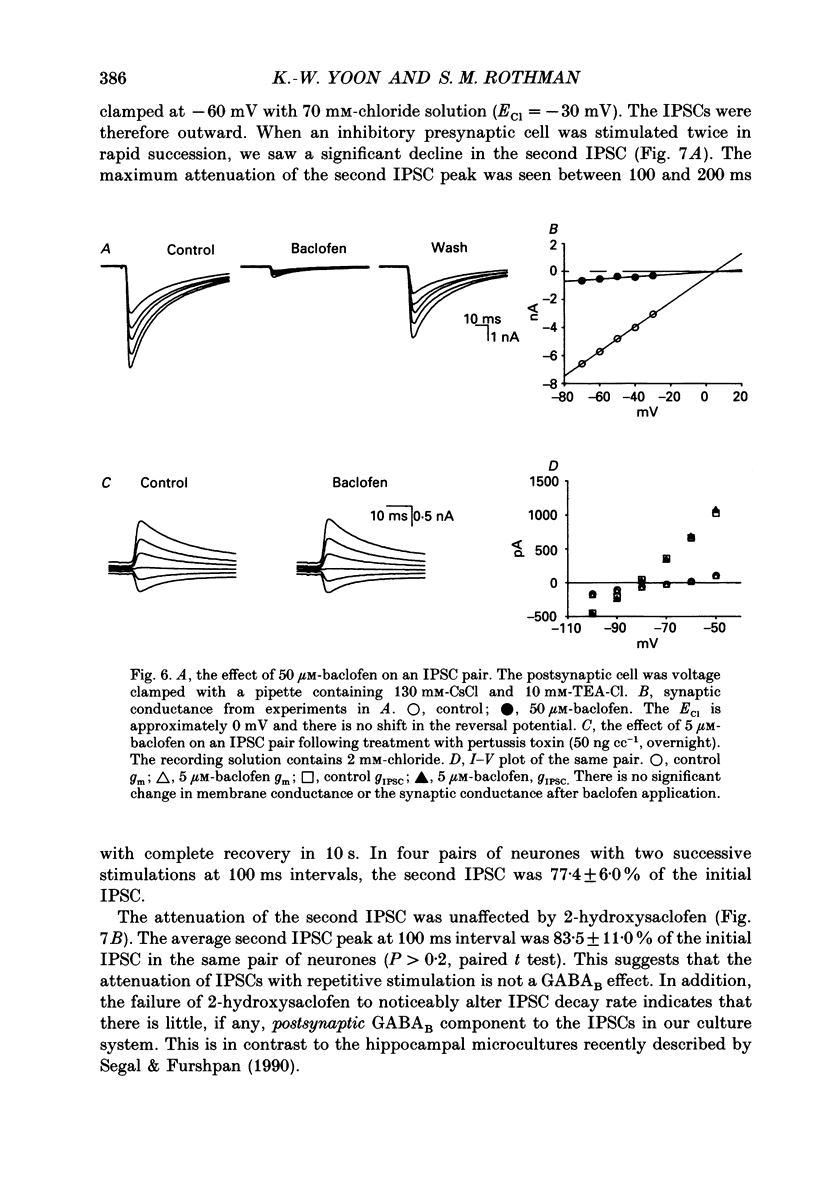
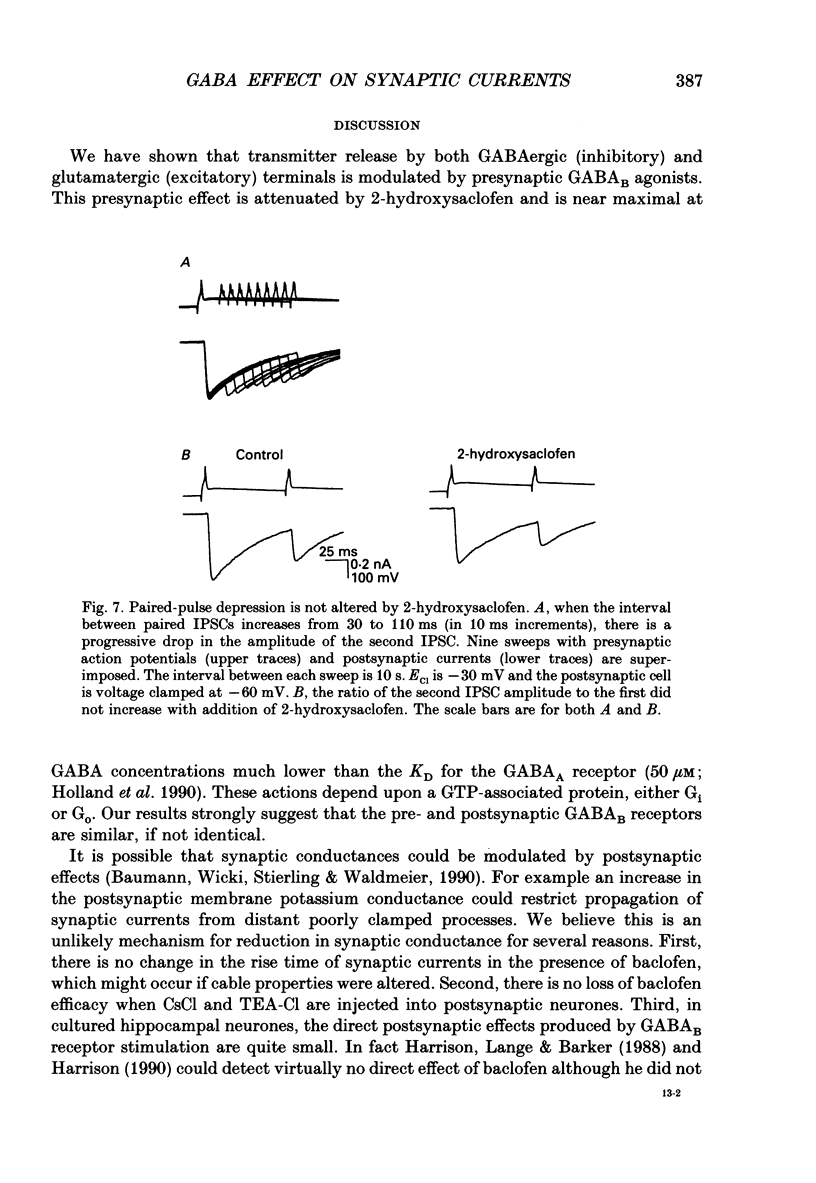
1. We examined the effects of gamma-aminobutyric acid (GABA) and baclofen on pre- and postsynaptic membrane conductances in dissociated rat hippocampal cells. Both GABA (5 microM with 10 microM-bicuculline) and baclofen (50 microM) caused small but significant increases in membrane conductance that were blocked by 2-hydroxysaclofen (100 microM), a GABAB receptor antagonist. This increase in membrane conductance seems to be mediated by GABAB receptors. 2. At a low concentration of GABA (1 microM) which has a very small direct postsynaptic effect on GABAA receptors, no postsynaptic GABAB effect was detected. However, at this concentration, GABA near maximally attenuated both excitatory and inhibitory synaptic currents. This GABA effect on transmitter release was significantly attenuated by 2-hydroxysaclofen. 3. Baclofen was also more potent in attenuating the inhibitory synaptic conductance than increasing postsynaptic conductance. Concentrations below 1 microM diminished synaptic currents by greater than 50%. At these low baclofen concentrations 2-hydroxysaclofen significantly attenuated baclofen's reduction of synaptic currents. 4. The effects of GABA and baclofen on synaptic conductances were blocked by pretreating the cultures with pertussis toxin, suggesting that a GTP-associated protein, Gi or Go is responsible for reducing transmitter release. 5. Despite the ability of GABA to diminish inhibitory synaptic currents through GABAB receptor activation, we observed no effect of 2-hydroxysaclofen on paired-pulse depression. Therefore, these presynaptic GABAB receptors may not be true 'autoreceptors'. 6. Our findings indicate that in culture, at least, the presynaptic GABAB effect responsible for synaptic modulation has a pharmacological profile similar to the postsynaptic GABAB effect. At present, it is unnecessary to postulate two different types of GABAB receptors.
Full text
PDF

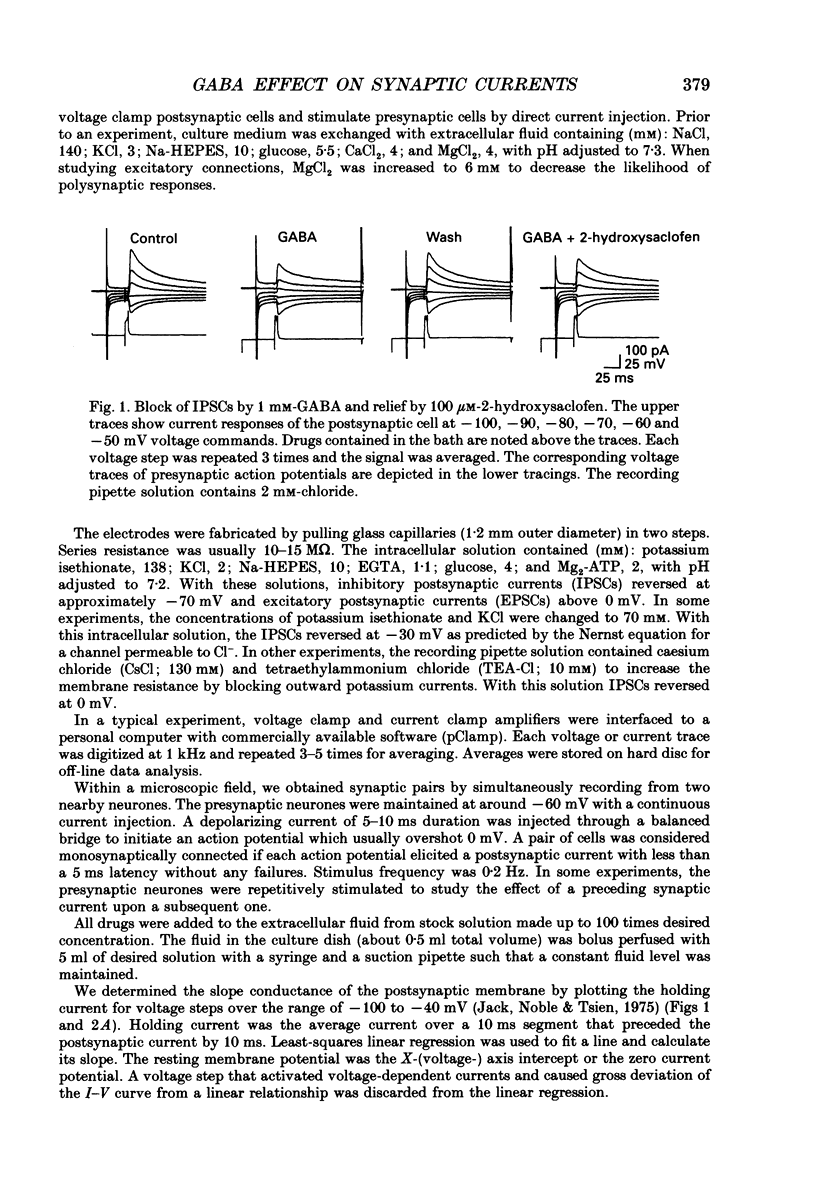
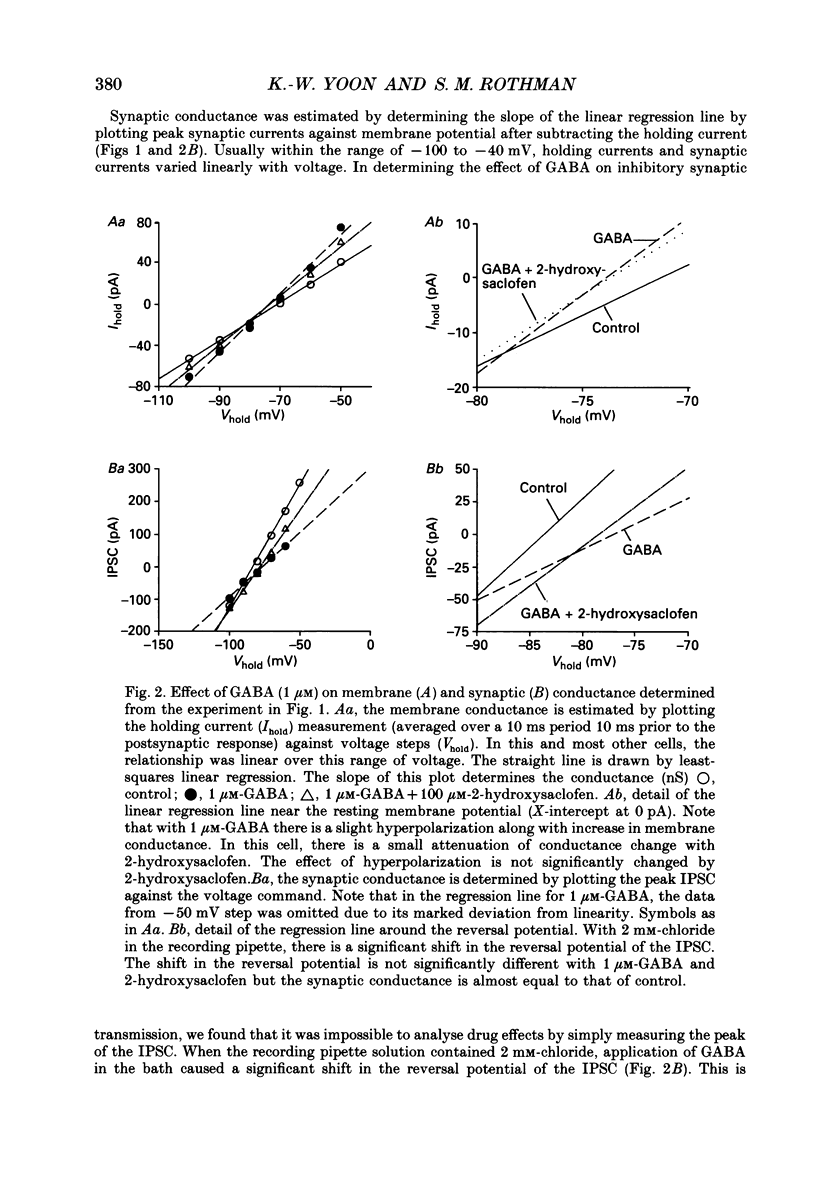

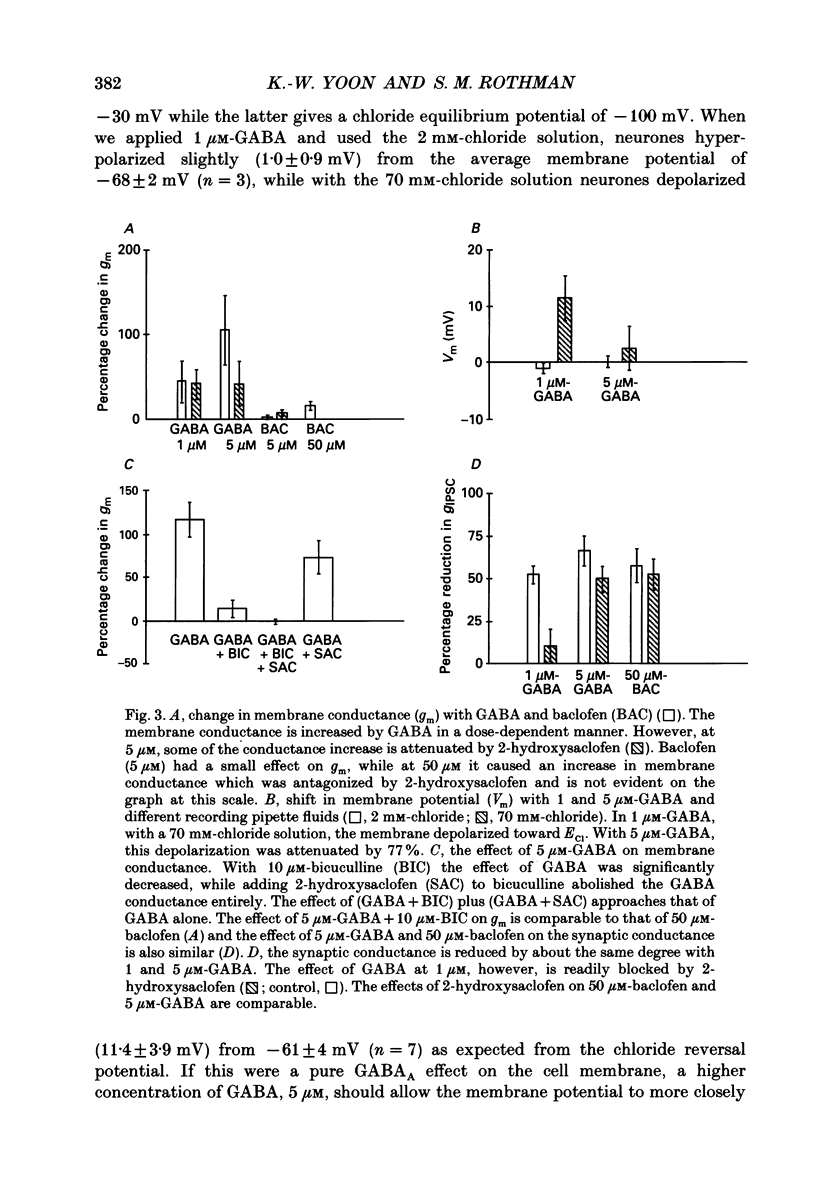
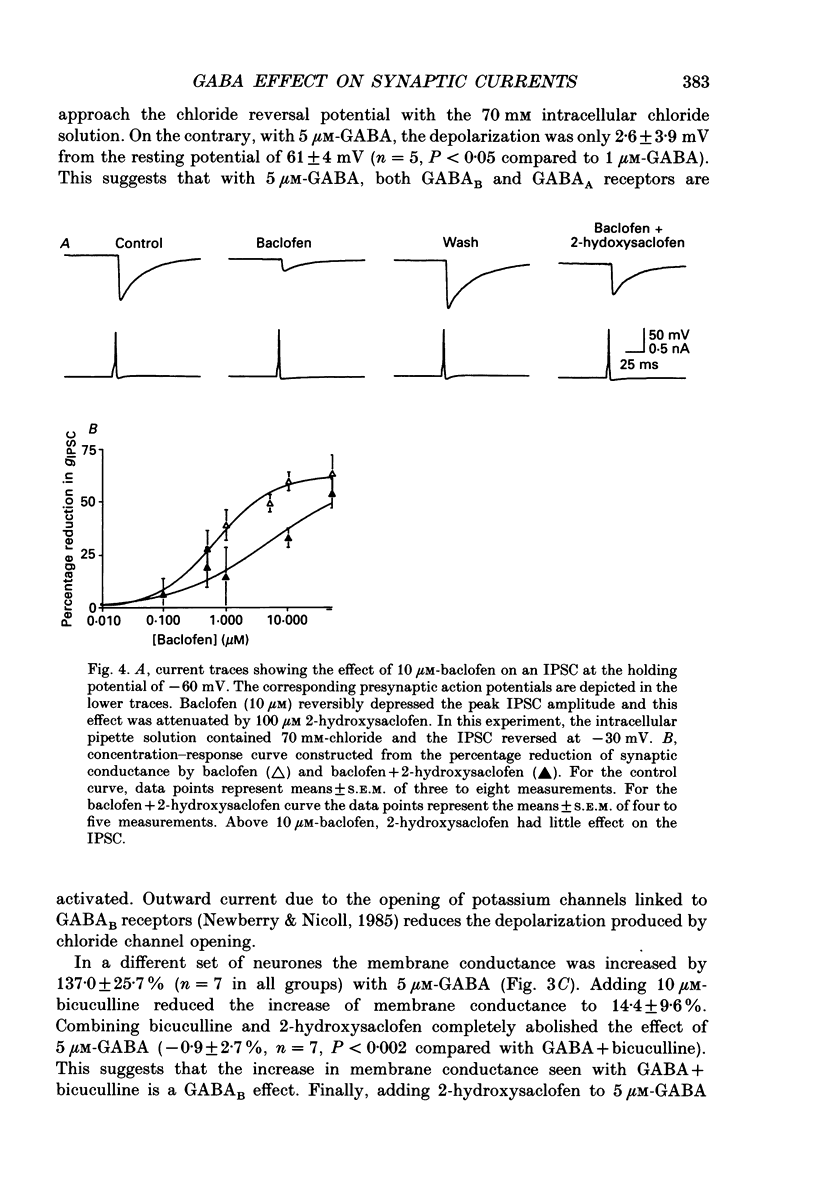




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann P. A., Wicki P., Stierlin C., Waldmeier P. C. Investigations on GABAB receptor-mediated autoinhibition of GABA release. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jan-Feb;341(1-2):88–93. doi: 10.1007/BF00195063. [DOI] [PubMed] [Google Scholar]
- Bowery N. GABAB receptors and their significance in mammalian pharmacology. Trends Pharmacol Sci. 1989 Oct;10(10):401–407. doi: 10.1016/0165-6147(89)90188-0. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Gynther B. D., Beattie D. T., Kerr D. I., Prager R. H. Baclofen antagonism by 2-hydroxy-saclofen in the cat spinal cord. Neurosci Lett. 1988 Sep 23;92(1):97–101. doi: 10.1016/0304-3940(88)90749-5. [DOI] [PubMed] [Google Scholar]
- Davies C. H., Davies S. N., Collingridge G. L. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990 May;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz R. A., Prince D. A. Frequency-dependent depression of inhibition in guinea-pig neocortex in vitro by GABAB receptor feed-back on GABA release. J Physiol. 1989 May;412:513–541. doi: 10.1113/jphysiol.1989.sp017629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C., Scott R. H. Inhibition of calcium currents in cultured rat dorsal root ganglion neurones by (-)-baclofen. Br J Pharmacol. 1986 May;88(1):213–220. doi: 10.1111/j.1476-5381.1986.tb09489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium ocmponent of sensory neurone action potentials. Nature. 1978 Dec 21;276(5690):837–839. doi: 10.1038/276837a0. [DOI] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. A physiological role for GABAB receptors in the central nervous system. Nature. 1988 Mar 10;332(6160):156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. Pre- and postsynaptic GABAB receptors in the hippocampus have different pharmacological properties. Neuron. 1988 Sep;1(7):585–591. doi: 10.1016/0896-6273(88)90108-0. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H., Brown D. A. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Lange G. D., Barker J. L. (-)-Baclofen activates presynaptic GABAB receptors on GABAergic inhibitory neurons from embryonic rat hippocampus. Neurosci Lett. 1988 Feb 15;85(1):105–109. doi: 10.1016/0304-3940(88)90437-5. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Lovinger D. M., Lambert N. A., Teyler T. J., Prager R., Ong J., Kerr D. I. The actions of 2-hydroxy-saclofen at presynaptic GABAB receptors in the rat hippocampus. Neurosci Lett. 1990 Nov 13;119(2):272–276. doi: 10.1016/0304-3940(90)90851-y. [DOI] [PubMed] [Google Scholar]
- Harrison N. L. On the presynaptic action of baclofen at inhibitory synapses between cultured rat hippocampal neurones. J Physiol. 1990 Mar;422:433–446. doi: 10.1113/jphysiol.1990.sp017993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Holland K. D., Ferrendelli J. A., Covey D. F., Rothman S. M. Physiological regulation of the picrotoxin receptor by gamma-butyrolactones and gamma-thiobutyrolactones in cultured hippocampal neurons. J Neurosci. 1990 Jun;10(6):1719–1727. doi: 10.1523/JNEUROSCI.10-06-01719.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard J. R., Alger B. E. Whole-cell voltage-clamp study of the fading of GABA-activated currents in acutely dissociated hippocampal neurons. J Neurophysiol. 1986 Jul;56(1):1–18. doi: 10.1152/jn.1986.56.1.1. [DOI] [PubMed] [Google Scholar]
- Kamatchi G. L., Ticku M. K. GABAB receptor activation inhibits Ca2(+)-activated 86Rb-efflux in cultured spinal cord neurons via G-protein mechanism. Brain Res. 1990 Jan 8;506(2):181–186. doi: 10.1016/0006-8993(90)91249-g. [DOI] [PubMed] [Google Scholar]
- Kerr D. I., Ong J., Johnston G. A., Abbenante J., Prager R. H. 2-Hydroxy-saclofen: an improved antagonist at central and peripheral GABAB receptors. Neurosci Lett. 1988 Sep 23;92(1):92–96. doi: 10.1016/0304-3940(88)90748-3. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988 Jul 29;241(4865):545–551. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- Peng Y. Y., Frank E. Activation of GABAB receptors causes presynaptic inhibition at synapses between muscle spindle afferents and motoneurons in the spinal cord of bullfrogs. J Neurosci. 1989 May;9(5):1502–1515. doi: 10.1523/JNEUROSCI.09-05-01502.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. M., Furshpan E. J. Epileptiform activity in microcultures containing small numbers of hippocampal neurons. J Neurophysiol. 1990 Nov;64(5):1390–1399. doi: 10.1152/jn.1990.64.5.1390. [DOI] [PubMed] [Google Scholar]
- Soltesz I., Haby M., Leresche N., Crunelli V. The GABAB antagonist phaclofen inhibits the late K+-dependent IPSP in cat and rat thalamic and hippocampal neurones. Brain Res. 1988 May 17;448(2):351–354. doi: 10.1016/0006-8993(88)91275-9. [DOI] [PubMed] [Google Scholar]
- Stirling J. M., Cross A. J., Robinson T. N., Green A. R. The effects of GABAB receptor agonists and antagonists on potassium-stimulated [Ca2+]i in rat brain synaptosomes. Neuropharmacology. 1989 Jul;28(7):699–704. doi: 10.1016/0028-3908(89)90153-6. [DOI] [PubMed] [Google Scholar]
- VanDongen A. M., Codina J., Olate J., Mattera R., Joho R., Birnbaumer L., Brown A. M. Newly identified brain potassium channels gated by the guanine nucleotide binding protein Go. Science. 1988 Dec 9;242(4884):1433–1437. doi: 10.1126/science.3144040. [DOI] [PubMed] [Google Scholar]
- Yamada K. A., Dubinsky J. M., Rothman S. M. Quantitative physiological characterization of a quinoxalinedione non-NMDA receptor antagonist. J Neurosci. 1989 Sep;9(9):3230–3236. doi: 10.1523/JNEUROSCI.09-09-03230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K. W., Rothman S. M. Adenosine inhibits excitatory but not inhibitory synaptic transmission in the hippocampus. J Neurosci. 1991 May;11(5):1375–1380. doi: 10.1523/JNEUROSCI.11-05-01375.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


