Abstract
1. The efficiency of the metathoracic tergosternal muscle of the locust Schistocerca americana was examined by simultaneously measuring work output from the muscle and oxygen consumption by the muscle. The work output was determined using the work-loop technique in which the muscle is subjected to periodic strain and to phasic stimulation in the strain cycle. The area of the loop formed by plotting muscle force against muscle length over a cycle is the net work output for that cycle. 2. The tergosternal muscle is a synchronous, parallel-fibred muscle containing two motor units with similar contraction kinetics. The average twitch rise time (30 degrees C) was 15 ms, the twitch duration (to 50% relaxation) was 26 ms, and the peak twitch tension with both units active was 73 kN m-2. The maximum mechanical power output during sinusoidal shortening at 20 Hz with both motor units active and stimulated once per cycle averaged 37 W kg-1. 3. The overall efficiency of the tergosternal muscle averaged 6.4% (range 4-10%) where efficiency is defined as the ratio of the net work done (20 Hz sinusoidal strain, 1 stimulus per cycle, optimum strain amplitude and stimulus phase) to the caloric equivalent of the oxygen consumed. The efficiency was independent of the duration of the test period (examined range = 10-30 s) and the same when both motor units were active as when only one was stimulated. 4. Stimulating the muscle with two stimuli per cycle (interstimulus interval = 6 ms) increased the work per cycle by about 13% above that with single stimuli per cycle, but the muscle fatigued more rapidly and after 15-25 s the power output was less with two stimuli per cycle than with one. The efficiency with two stimuli per cycle was slightly less than that with one shock per cycle. 5. The oxygen consumption during normal work cycles at 20 Hz with optimum stimulus phase and strain was greater by about 15% than the oxygen consumption during isometric contractions at the same frequency.
Full text
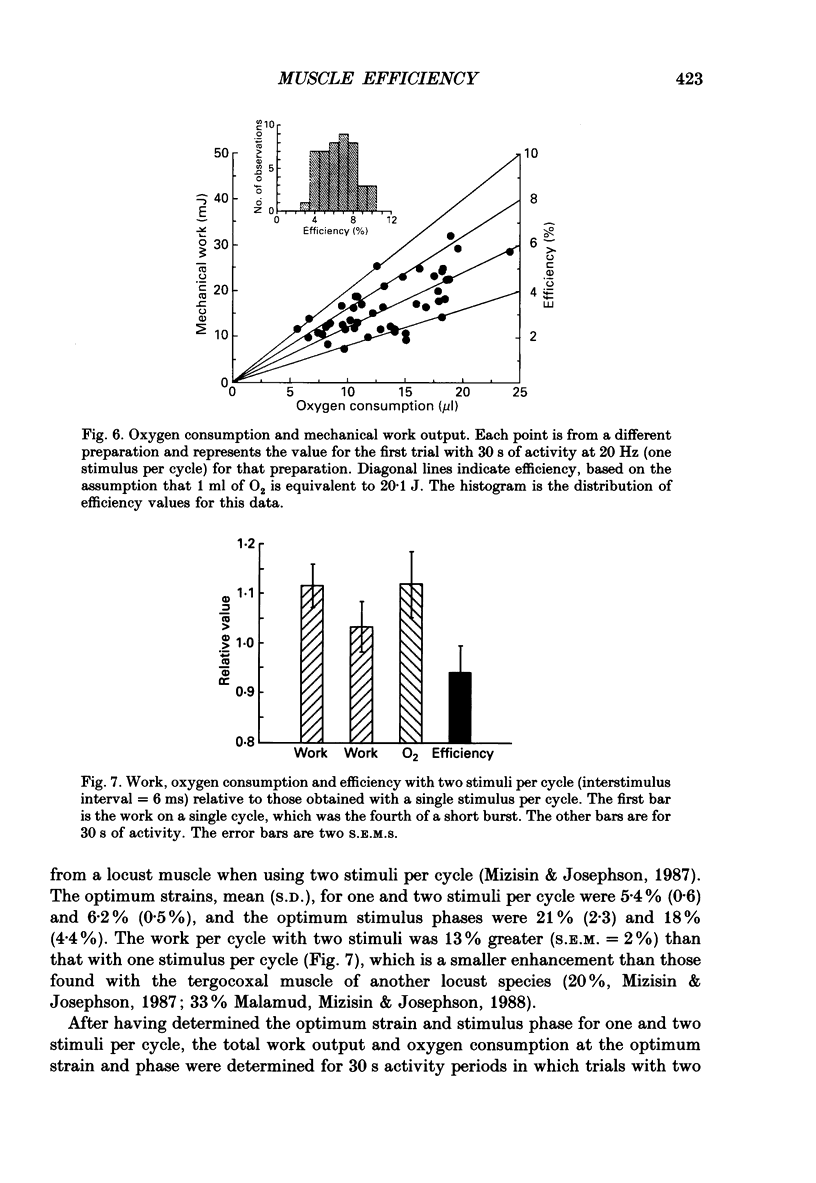
PDF



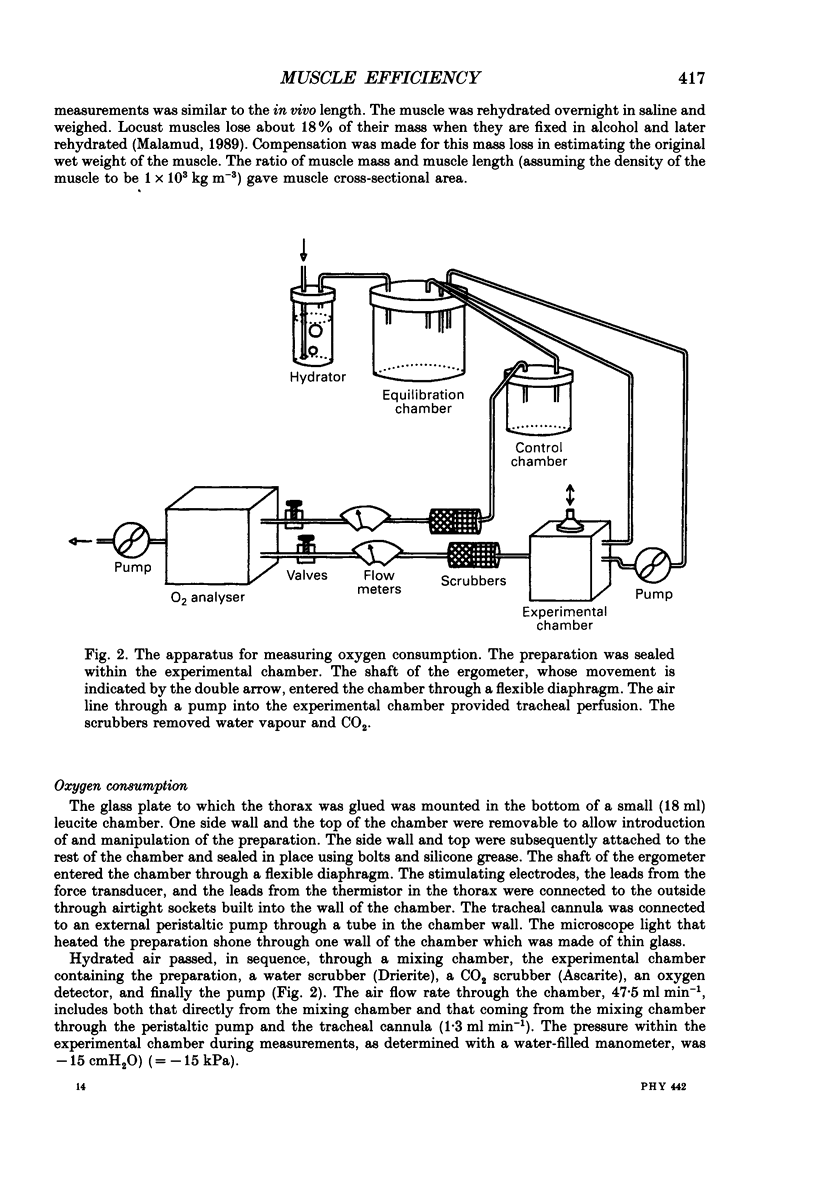

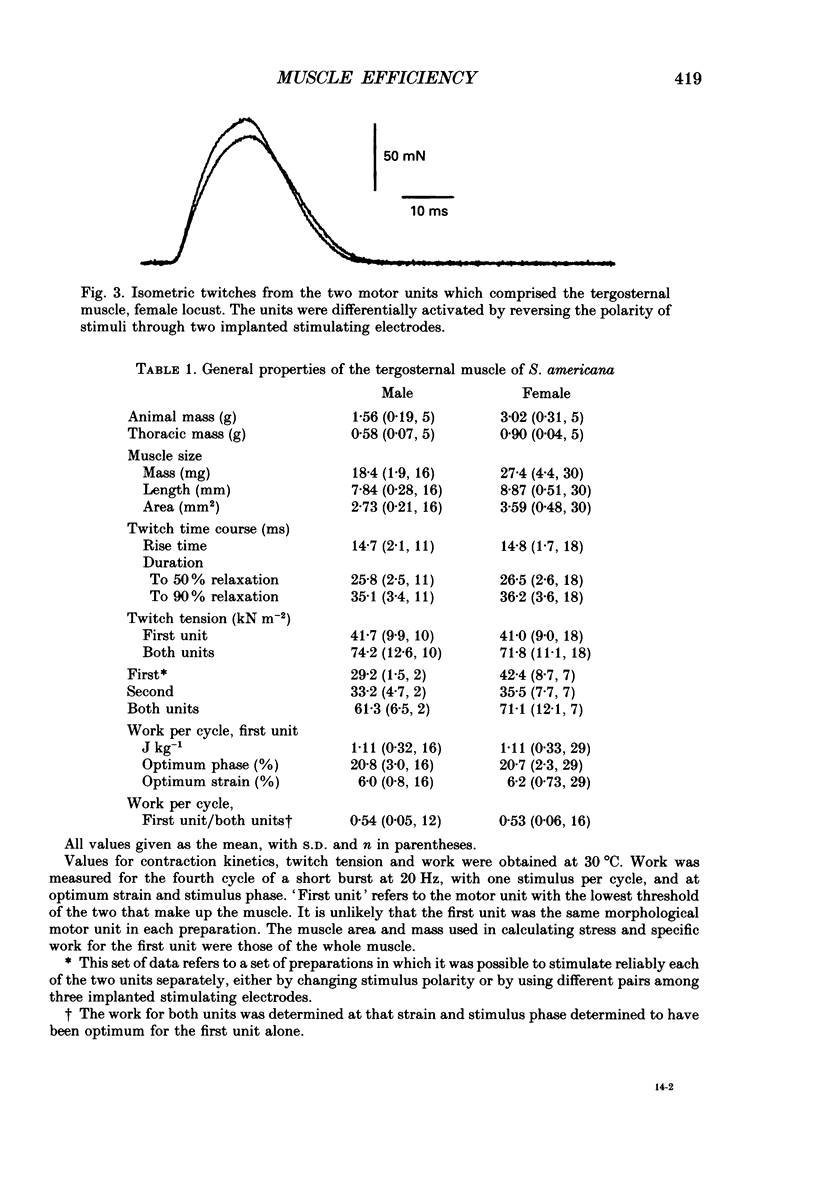
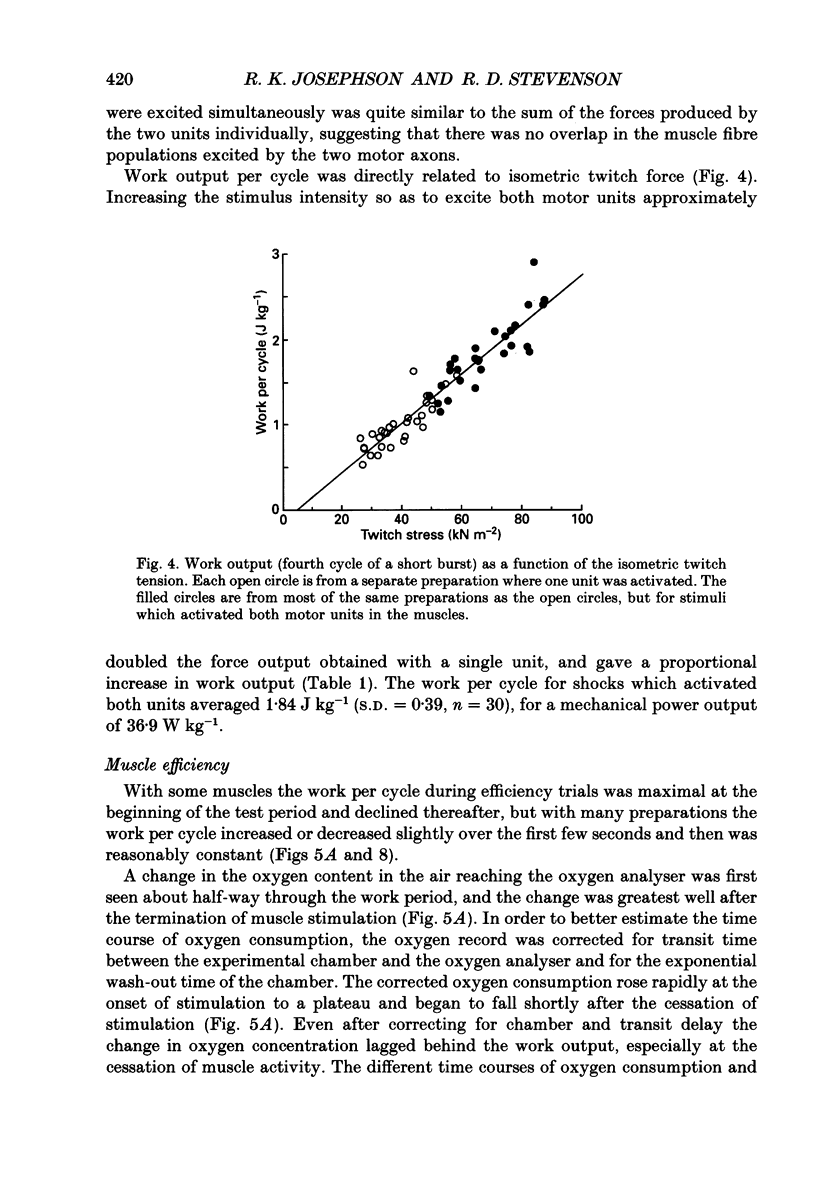
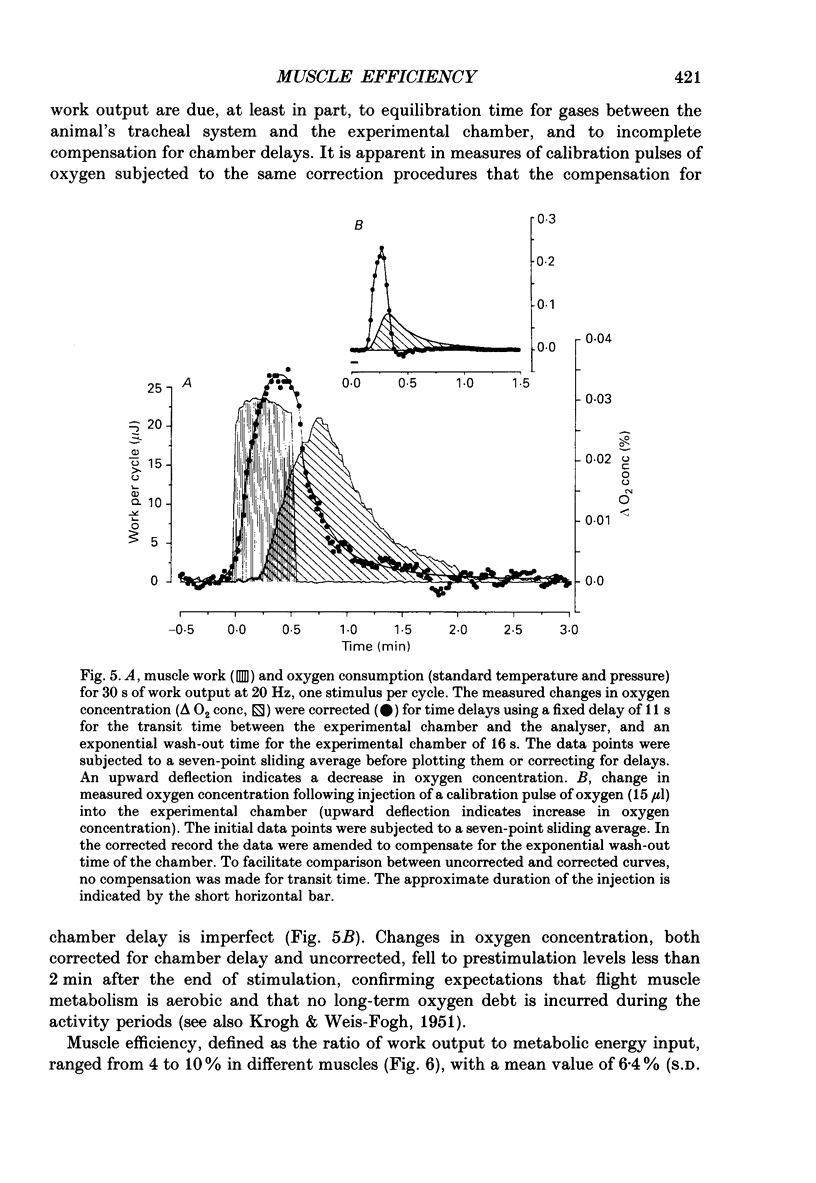

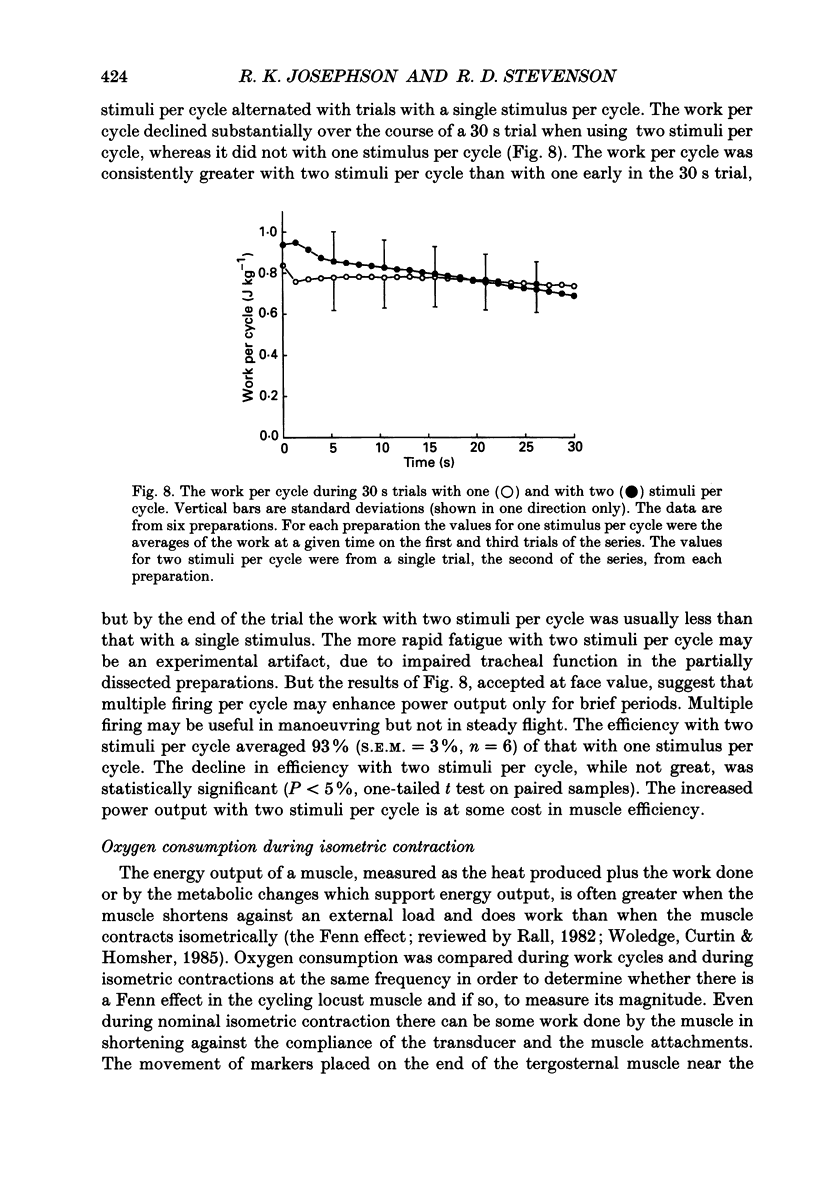




Selected References
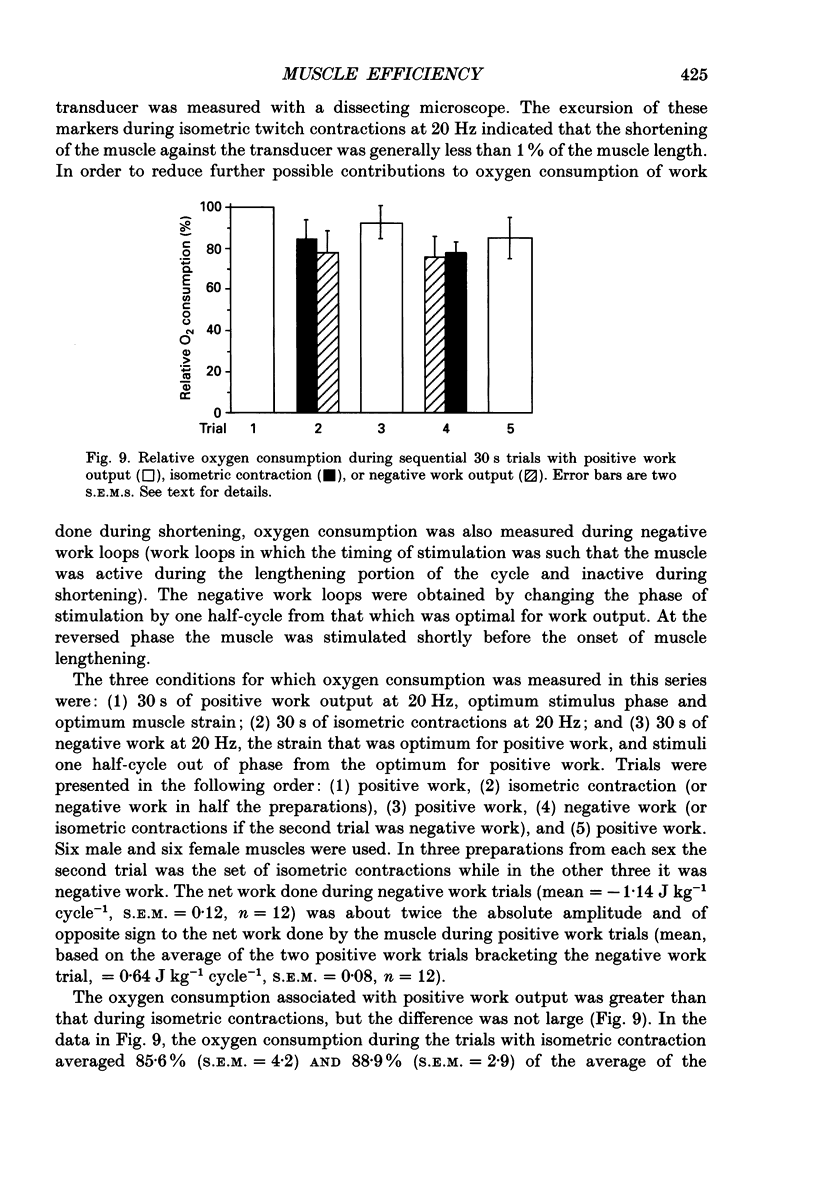
These references are in PubMed. This may not be the complete list of references from this article.
- Burchfield D. M., Rall J. A. Energetics of activation in frog skeletal muscle at sarcomere lengths beyond myofilament overlap. Biophys J. 1985 Dec;48(6):1049–1051. doi: 10.1016/S0006-3495(85)83867-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haan A., Van Ingen Schenau G. J., Ettema G. J., Huijing P. A., Lodder M. A. Efficiency of rat medial gastrocnemius muscle in contractions with and without an active prestretch. J Exp Biol. 1989 Jan;141:327–341. doi: 10.1242/jeb.141.1.327. [DOI] [PubMed] [Google Scholar]
- Ellington C. P. Power and efficiency of insect flight muscle. J Exp Biol. 1985 Mar;115:293–304. doi: 10.1242/jeb.115.1.293. [DOI] [PubMed] [Google Scholar]
- Gibbs C. L., Gibson W. R. Energy production of rat soleus muscle. Am J Physiol. 1972 Oct;223(4):864–871. doi: 10.1152/ajplegacy.1972.223.4.864. [DOI] [PubMed] [Google Scholar]
- Heglund N. C., Cavagna G. A. Mechanical work, oxygen consumption, and efficiency in isolated frog and rat muscle. Am J Physiol. 1987 Jul;253(1 Pt 1):C22–C29. doi: 10.1152/ajpcell.1987.253.1.C22. [DOI] [PubMed] [Google Scholar]
- Heglund N. C., Taylor C. R. Speed, stride frequency and energy cost per stride: how do they change with body size and gait? J Exp Biol. 1988 Sep;138:301–318. doi: 10.1242/jeb.138.1.301. [DOI] [PubMed] [Google Scholar]
- Homsher E., Kean C. J. Skeletal muscle energetics and metabolism. Annu Rev Physiol. 1978;40:93–131. doi: 10.1146/annurev.ph.40.030178.000521. [DOI] [PubMed] [Google Scholar]
- Homsher E., Mommaerts W. F., Ricchiuti N. V., Wallner A. Activation heat, activation metabolism and tension-related heat in frog semitendinosus muscles. J Physiol. 1972 Feb;220(3):601–625. doi: 10.1113/jphysiol.1972.sp009725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick M. J., Davies R. E. The chemical energetics of muscle contraction. II. The chemistry, efficiency and power of maximally working sartorius muscles. Appendix. Free energy and enthalpy of atp hydrolysis in the sarcoplasm. Proc R Soc Lond B Biol Sci. 1969 Dec 23;174(1036):315–353. doi: 10.1098/rspb.1969.0096. [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Larson R. E., Davies R. E. The chemical energetics of muscle contraction. I. Activation heat, heat of shortening and ATP utilization for activation-relaxation processes. Proc R Soc Lond B Biol Sci. 1969 Dec 23;174(1036):293–313. doi: 10.1098/rspb.1969.0095. [DOI] [PubMed] [Google Scholar]
- Rall J. A. Dependence of energy output on force generation during muscle contraction. Am J Physiol. 1978 Jul;235(1):C20–C24. doi: 10.1152/ajpcell.1978.235.1.C20. [DOI] [PubMed] [Google Scholar]
- Rall J. A. Energetics of Ca2+ cycling during skeletal muscle contraction. Fed Proc. 1982 Feb;41(2):155–160. [PubMed] [Google Scholar]
- Rall J. A., Schottelius B. A. Energetics of contraction in phasic and tonic skeletal muscles of the chicken. J Gen Physiol. 1973 Sep;62(3):303–323. doi: 10.1085/jgp.62.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. C. Energetics of activation in frog and toad muscle. J Physiol. 1972 Feb;220(3):583–599. doi: 10.1113/jphysiol.1972.sp009724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainbsy W. N., Gladden L. B., Barclay J. K., Wilson B. A. Exercise efficiency: validity of base-line subtractions. J Appl Physiol Respir Environ Exerc Physiol. 1980 Mar;48(3):518–522. doi: 10.1152/jappl.1980.48.3.518. [DOI] [PubMed] [Google Scholar]
- Wendt I. R., Gibbs C. L. Energy production of rat extensor digitorum longus muscle. Am J Physiol. 1973 May;224(5):1081–1086. doi: 10.1152/ajplegacy.1973.224.5.1081. [DOI] [PubMed] [Google Scholar]


