Abstract
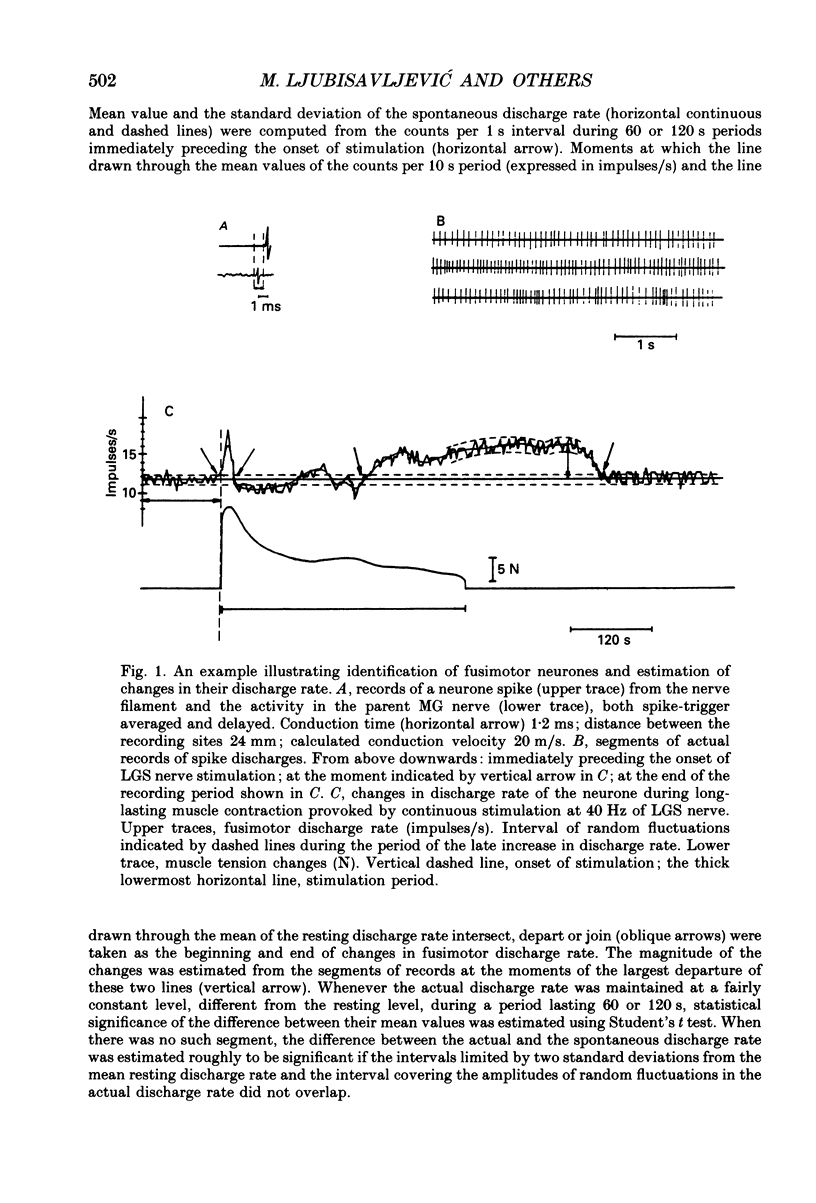
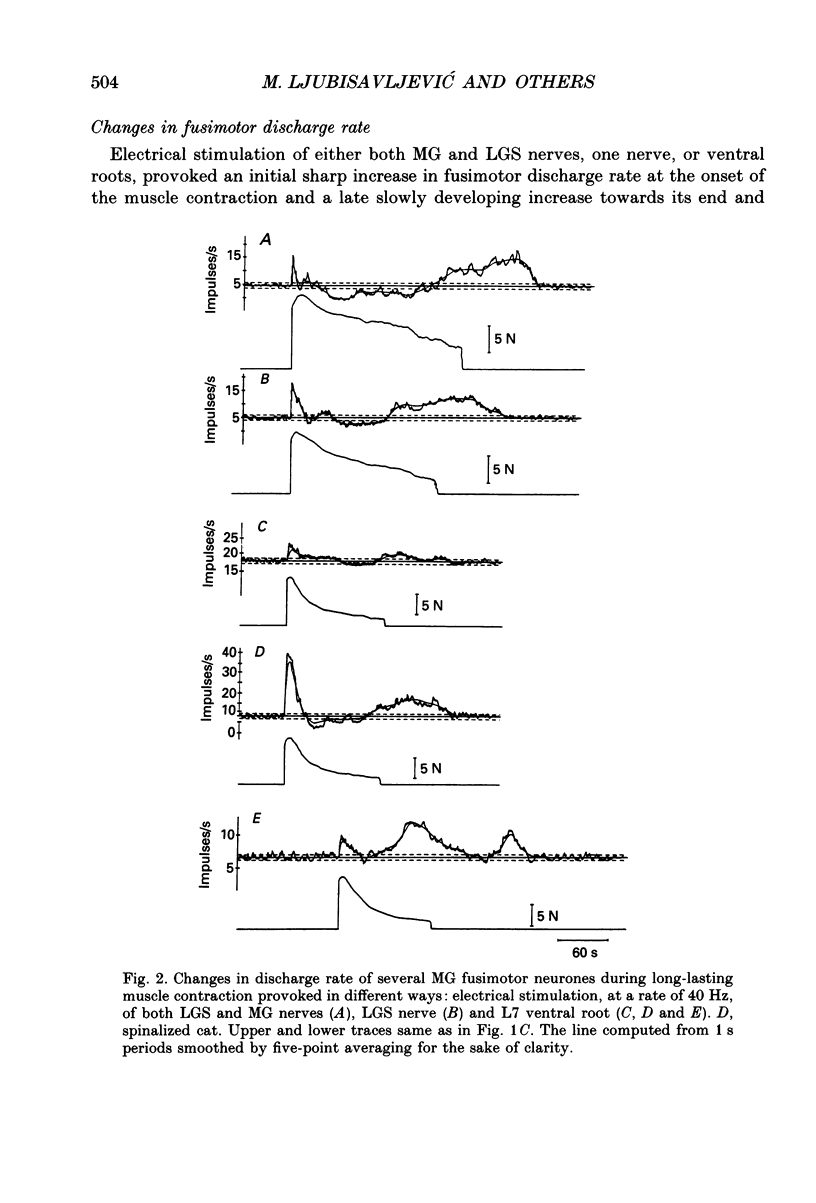
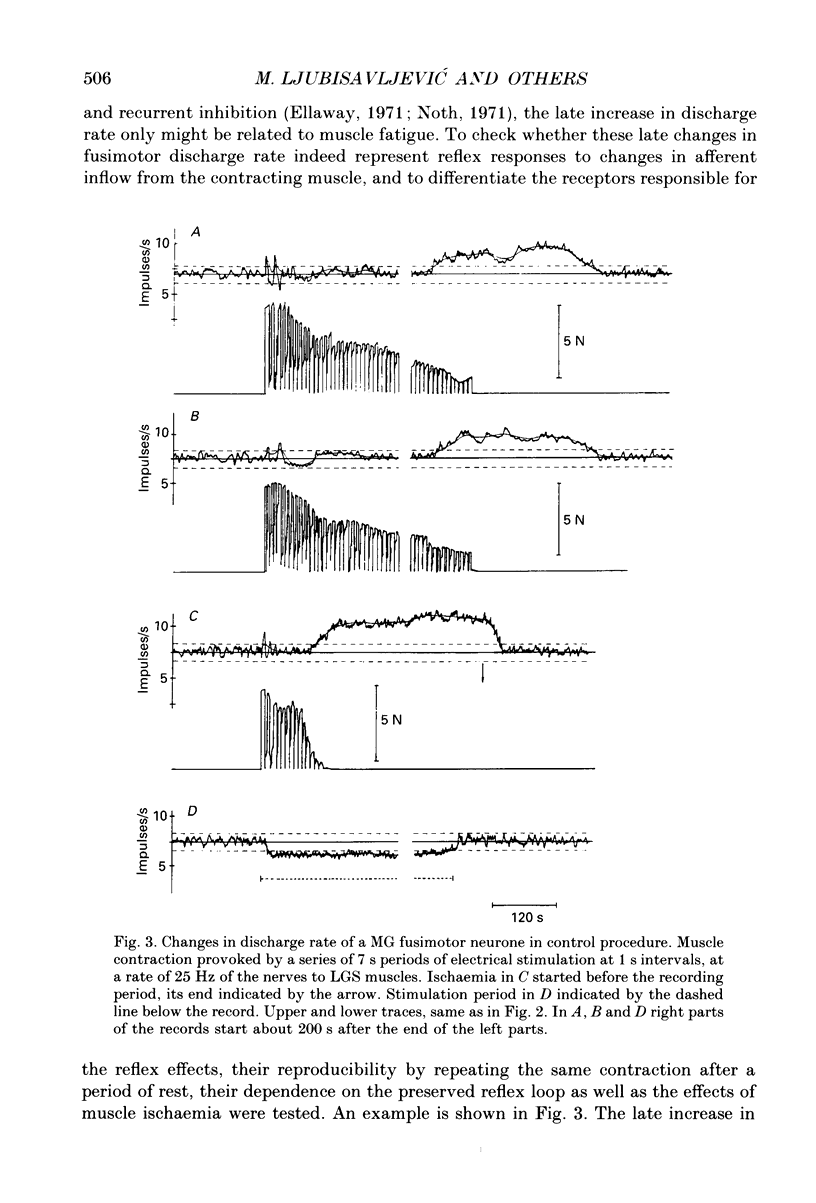
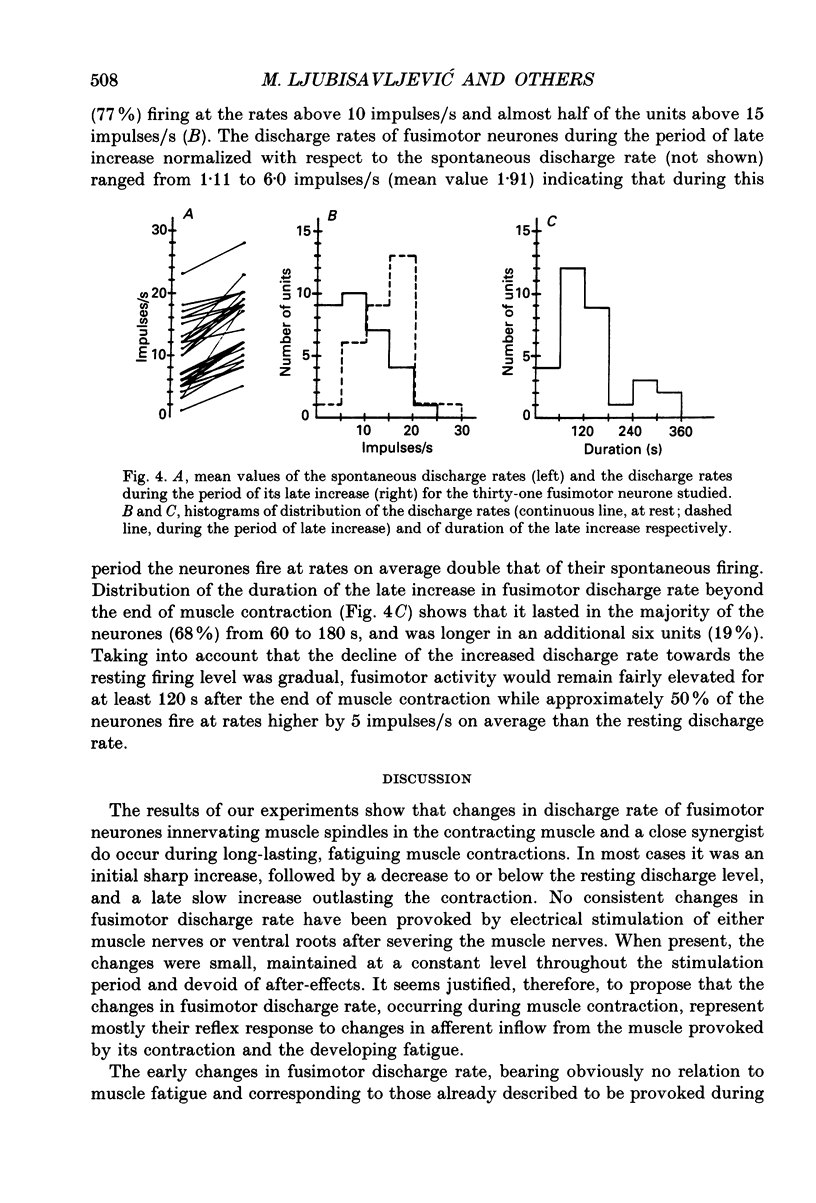
1. Changes in discharge rate of thirty-one fusimotor neurones to triceps surae muscles during long-lasting, fatiguing contractions of these muscles were studied in decerebrate cats. Discharges of fusimotor neurones were recorded from the nerve filaments. Muscle contractions were elicited by electrical stimulation of either the muscle nerves (twenty-one neurones) or the corresponding ventral roots (ten neurones), until the muscle tension fell to about 30% of its initial value. 2. Early and late changes could be recognized in fusimotor discharge rate during long-lasting muscle contraction. The early changes obviously not related to muscle fatigue, consisted of an initial increase at the onset of muscle contraction and a subsequent decrease to or below the resting discharge level. The late change in discharge rate, supposedly related to muscle fatigue, was an increase developing gradually towards the end of muscle contraction, ranging at its peak from 2 to 15 impulses/s (mean value 5.5 impulses/s, n = 31) and outlasting the contraction for 20-320 s. 3. When the contracting muscle was made ischaemic the late increase in fusimotor discharge rate started earlier and was maintained until the arterial clamp was removed. After severing the muscle nerves distal to the site of stimulation no changes, a slight sustained increase, or else a decrease in fusimotor discharge rate occurred during electrical stimulation of either muscle nerves or ventral roots. At its cessation the spontaneous firing rate was reassumed immediately. Stimulation of the distal stumps of the severed nerves elicited no changes in fusimotor discharge rate. 4. It is proposed that the late increase in fusimotor discharge rate may appear due to autogenetic excitation of fusimotor neurones by discharge from group III and IV muscle afferent fibres provoked and/or enhanced by metabolic products liberated in muscle tissue during the fatiguing contraction. The fusimotor firing was estimated to remain elevated to a level twice that of the spontaneous activity on average for approximately 120 s after the muscle contraction. Its functional role in muscle fatigue is discussed.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anastasijević R., Jocić M., Vuco J. Discharge rate and reflex responses of fusimotor neurons during muscle ischemia. Exp Neurol. 1987 Aug;97(2):340–344. doi: 10.1016/0014-4886(87)90094-x. [DOI] [PubMed] [Google Scholar]
- Anastasijević R., Vuco J. The relative dependence of the activity of Renshaw cells on recurrent pathways during contraction of the triceps muscle. Pflugers Arch. 1978 Nov 30;377(3):255–261. doi: 10.1007/BF00584281. [DOI] [PubMed] [Google Scholar]
- Appelberg B., Hulliger M., Johansson H., Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of group II muscle afferent fibres in the hind limb of the cat. J Physiol. 1983 Feb;335:255–273. doi: 10.1113/jphysiol.1983.sp014532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B., Hulliger M., Johansson H., Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of group III muscle afferent fibres in the hind limb of the cat. J Physiol. 1983 Feb;335:275–292. doi: 10.1113/jphysiol.1983.sp014533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberich P., Hoheisel U., Mense S. Effects of a carrageenan-induced myositis on the discharge properties of group III and IV muscle receptors in the cat. J Neurophysiol. 1988 May;59(5):1395–1409. doi: 10.1152/jn.1988.59.5.1395. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B. R., Dawson N. J., Johansson R. S., Lippold O. C. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol. 1986 Oct;379:451–459. doi: 10.1113/jphysiol.1986.sp016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongiovanni L. G., Hagbarth K. E. Tonic vibration reflexes elicited during fatigue from maximal voluntary contractions in man. J Physiol. 1990 Apr;423:1–14. doi: 10.1113/jphysiol.1990.sp018007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H., Sears T. A. Continuous conduction in demyelinated mammalian nerve fibers. Nature. 1976 Oct 28;263(5580):786–787. doi: 10.1038/263786a0. [DOI] [PubMed] [Google Scholar]
- Duchateau J., de Montigny L., Hainaut K. Electro-mechanical failures and lactate production during fatigue. Eur J Appl Physiol Occup Physiol. 1987;56(3):287–291. doi: 10.1007/BF00690894. [DOI] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Supraspinal control of interneurones mediating spinal reflexes. J Physiol. 1959 Oct;147:565–584. doi: 10.1113/jphysiol.1959.sp006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Murphy P. R. Autogenetic effects of muscle contraction on extensor gamma motoneurones in the cat. Exp Brain Res. 1980 Feb;38(3):305–312. doi: 10.1007/BF00236650. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H., Murphy P. R., Tripathi A. Closely coupled excitation of gamma-motoneurones by group III Muscle afferents with low mechanical threshold in the cat. J Physiol. 1982 Oct;331:481–498. doi: 10.1113/jphysiol.1982.sp014385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H. Recurrent inhibition of fusimotor neurones exhibiting background discharges in the decerebrate and the spinal cat. J Physiol. 1971 Jul;216(2):419–439. doi: 10.1113/jphysiol.1971.sp009533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz M., Mense S. Muscle receptors with group IV afferent fibres responding to application of bradykinin. Brain Res. 1975 Jul 18;92(3):369–383. doi: 10.1016/0006-8993(75)90323-6. [DOI] [PubMed] [Google Scholar]
- Garland S. J., Garner S. H., McComas A. J. Reduced voluntary electromyographic activity after fatiguing stimulation of human muscle. J Physiol. 1988 Jul;401:547–556. doi: 10.1113/jphysiol.1988.sp017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory J. E., Kenins P., Proske U. Can lactate-evoked cardiovascular responses be used to identify muscle ergoreceptors? Brain Res. 1987 Feb 24;404(1-2):375–378. doi: 10.1016/0006-8993(87)91398-9. [DOI] [PubMed] [Google Scholar]
- HUNT C. C. Relation of function to diameter in afferent fibers of muscle nerves. J Gen Physiol. 1954 Sep 20;38(1):117–131. doi: 10.1085/jgp.38.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward L., Breitbach D., Rymer W. Z. Increased inhibitory effects on close synergists during muscle fatigue in the decerebrate cat. Brain Res. 1988 Feb 2;440(1):199–203. doi: 10.1016/0006-8993(88)91178-x. [DOI] [PubMed] [Google Scholar]
- Hutton R. S., Nelson D. L. Stretch sensitivity of Golgi tendon organs in fatigued gastrocnemius muscle. Med Sci Sports Exerc. 1986 Feb;18(1):69–74. [PubMed] [Google Scholar]
- Hutton R. S., Smith J. L., Eldred E. Persisting changes in sensory and motor activity of a muscle following its reflex activation. Pflugers Arch. 1975;353(4):327–336. doi: 10.1007/BF00587029. [DOI] [PubMed] [Google Scholar]
- Johansson H., Sjölander P., Sojka P., Wadell I. Effects of electrical and natural stimulation of skin afferents on the gamma-spindle system of the triceps surae muscle. Neurosci Res. 1989 Aug;6(6):537–555. doi: 10.1016/0168-0102(89)90043-6. [DOI] [PubMed] [Google Scholar]
- Jovanović K., Anastasijević R., Vuco J. Reflex effects on gamma fusimotor neurones of chemically induced discharges in small-diameter muscle afferents in decerebrate cats. Brain Res. 1990 Jun 25;521(1-2):89–94. doi: 10.1016/0006-8993(90)91528-o. [DOI] [PubMed] [Google Scholar]
- Juel C., Bangsbo J., Graham T., Saltin B. Lactate and potassium fluxes from human skeletal muscle during and after intense, dynamic, knee extensor exercise. Acta Physiol Scand. 1990 Oct;140(2):147–159. doi: 10.1111/j.1748-1716.1990.tb08986.x. [DOI] [PubMed] [Google Scholar]
- Kaufman M. P., Rybicki K. J., Waldrop T. G., Ordway G. A. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol Respir Environ Exerc Physiol. 1984 Sep;57(3):644–650. doi: 10.1152/jappl.1984.57.3.644. [DOI] [PubMed] [Google Scholar]
- Llewellyn M., Yang J. F., Prochazka A. Human H-reflexes are smaller in difficult beam walking than in normal treadmill walking. Exp Brain Res. 1990;83(1):22–28. doi: 10.1007/BF00232189. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B., RUSHWORTH G. The discharge from muscle spindles as an indicator of gamma efferent paralysis by procaine. J Physiol. 1958 Mar 11;140(3):421–426. [PMC free article] [PubMed] [Google Scholar]
- Mense S., Stahnke M. Responses in muscle afferent fibres of slow conduction velocity to contractions and ischaemia in the cat. J Physiol. 1983 Sep;342:383–397. doi: 10.1113/jphysiol.1983.sp014857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. L., Hutton R. S. Dynamic and static stretch responses in muscle spindle receptors in fatigued muscle. Med Sci Sports Exerc. 1985 Aug;17(4):445–450. doi: 10.1249/00005768-198508000-00007. [DOI] [PubMed] [Google Scholar]
- Noth J., Thilmann A. Autogenetic excitation of extensor gamma-motoneurones by group II muscle afferents in the cat. Neurosci Lett. 1980 Apr;17(1-2):23–26. doi: 10.1016/0304-3940(80)90055-5. [DOI] [PubMed] [Google Scholar]
- Piercey M. F., Goldfarb J. Discharge patterns of Renshaw cells evoked by volleys in ipsilateral cutaneous and high-threshold muscle afferents and their relationship to reflexes recorded in ventral roots. J Neurophysiol. 1974 Mar;37(2):294–302. doi: 10.1152/jn.1974.37.2.294. [DOI] [PubMed] [Google Scholar]
- Rotto D. M., Kaufman M. P. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol (1985) 1988 Jun;64(6):2306–2313. doi: 10.1152/jappl.1988.64.6.2306. [DOI] [PubMed] [Google Scholar]
- Rotto D. M., Schultz H. D., Longhurst J. C., Kaufman M. P. Sensitization of group III muscle afferents to static contraction by arachidonic acid. J Appl Physiol (1985) 1990 Mar;68(3):861–867. doi: 10.1152/jappl.1990.68.3.861. [DOI] [PubMed] [Google Scholar]
- Rymer W. Z., Houk J. C., Crago P. E. Mechanisms of the clasp-knife reflex studied in an animal model. Exp Brain Res. 1979 Sep;37(1):93–113. doi: 10.1007/BF01474257. [DOI] [PubMed] [Google Scholar]
- Thimm F., Baum K. Response of chemosensitive nerve fibers of group III and IV to metabolic changes in rat muscles. Pflugers Arch. 1987 Sep;410(1-2):143–152. doi: 10.1007/BF00581907. [DOI] [PubMed] [Google Scholar]


