Abstract
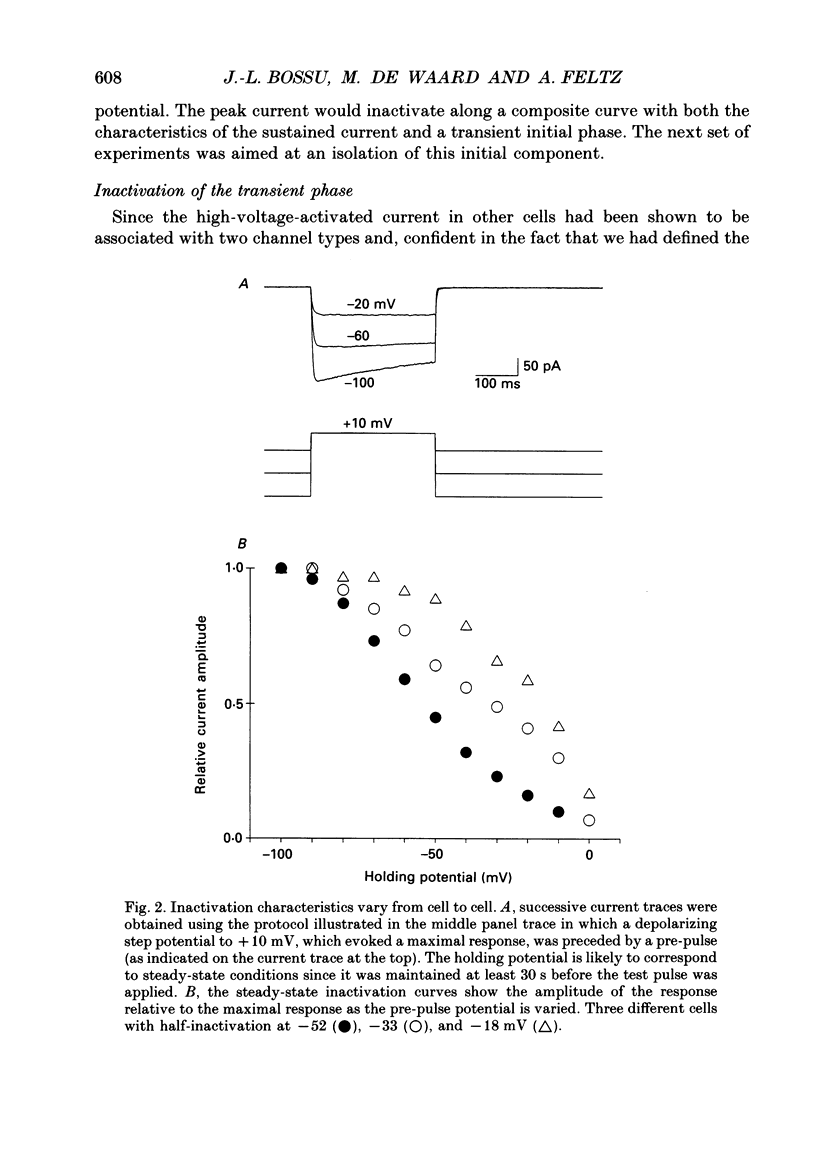
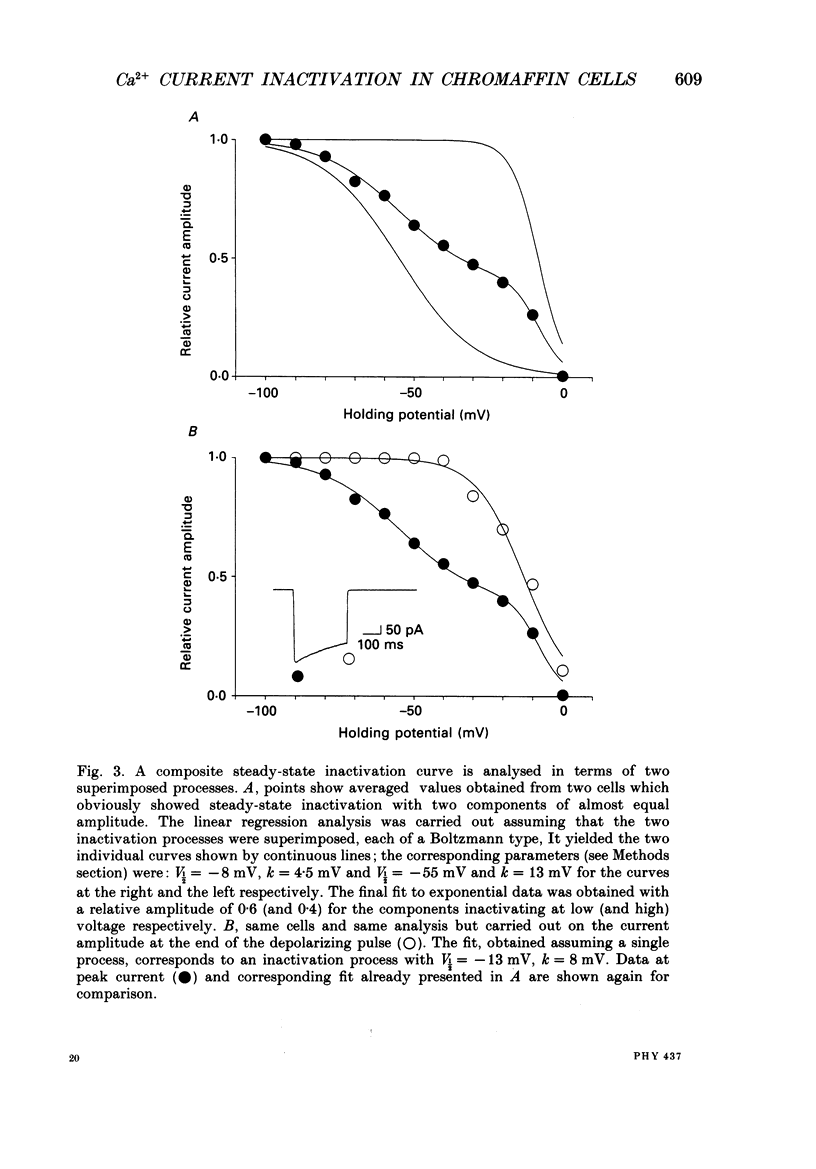
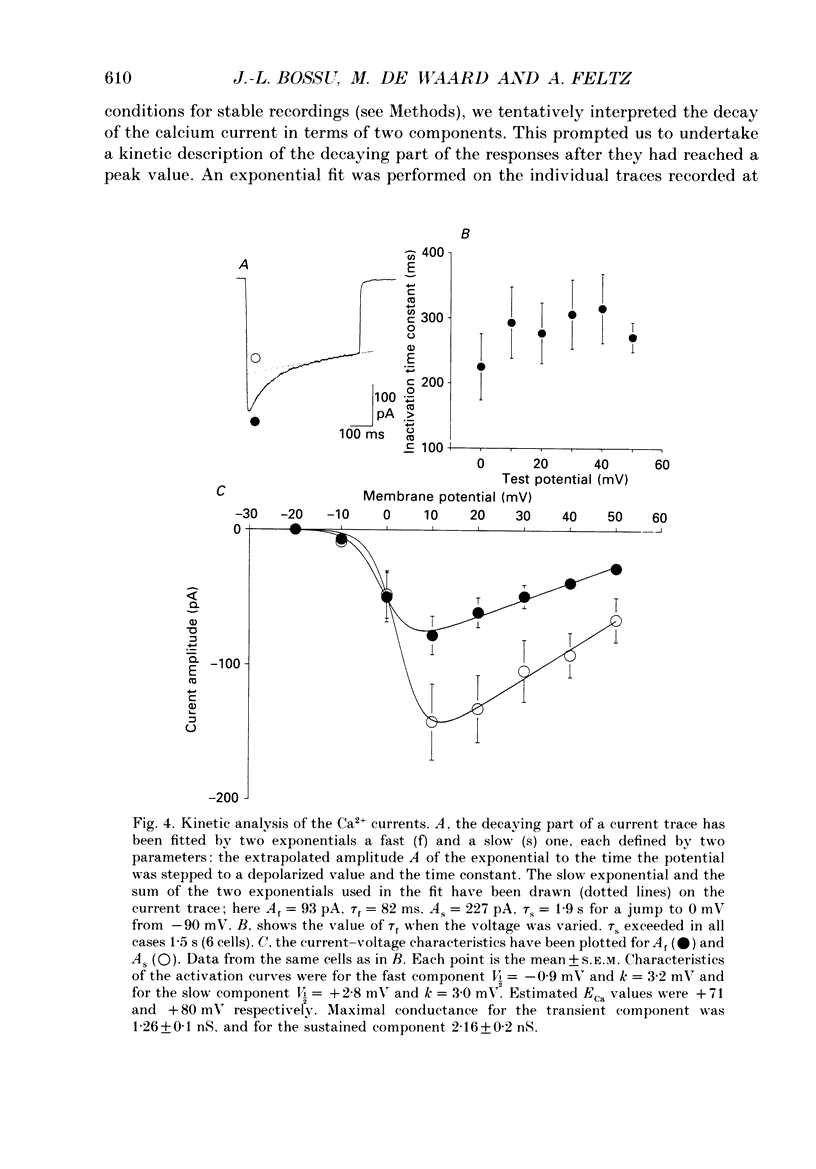
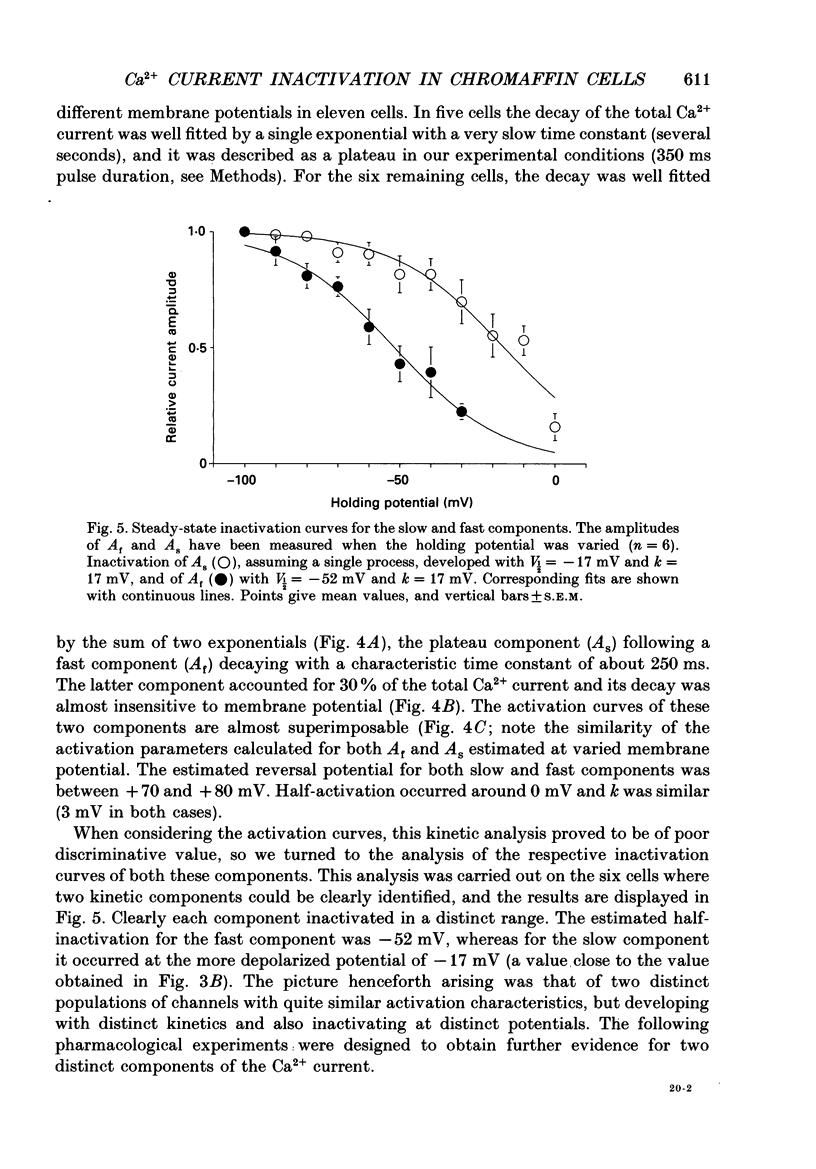
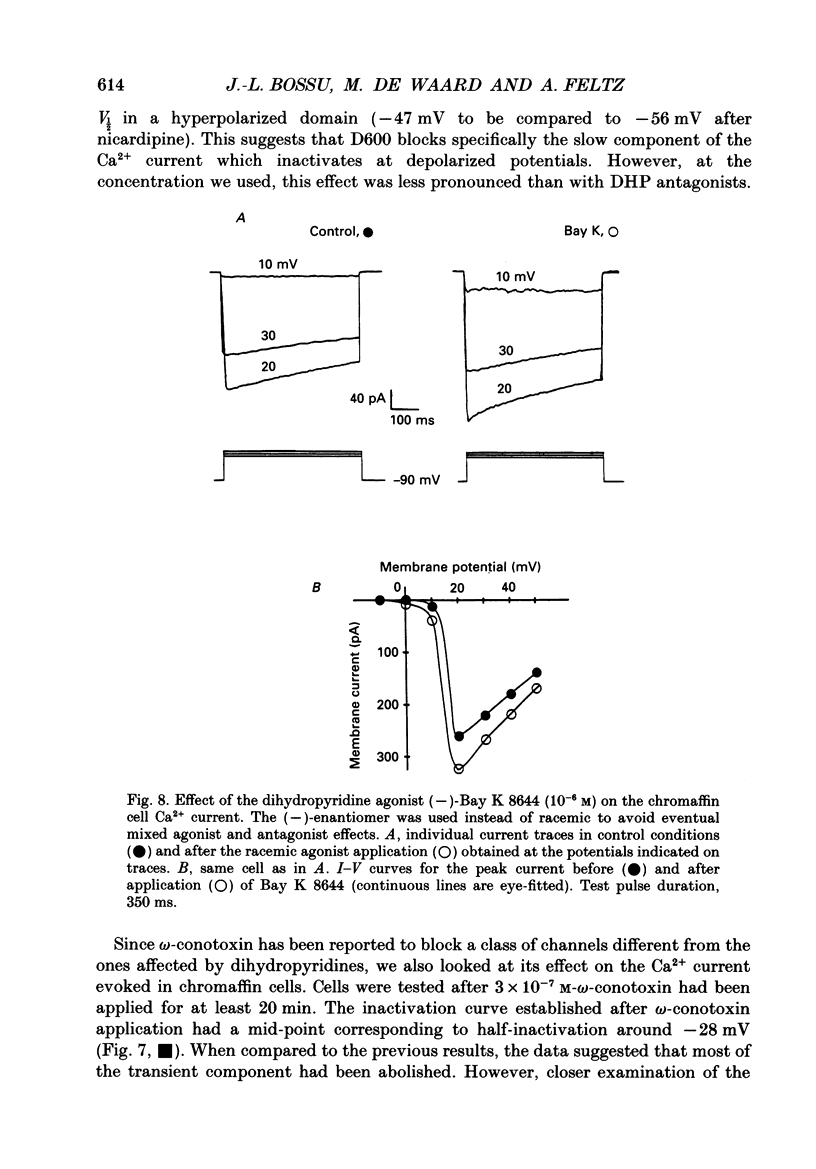
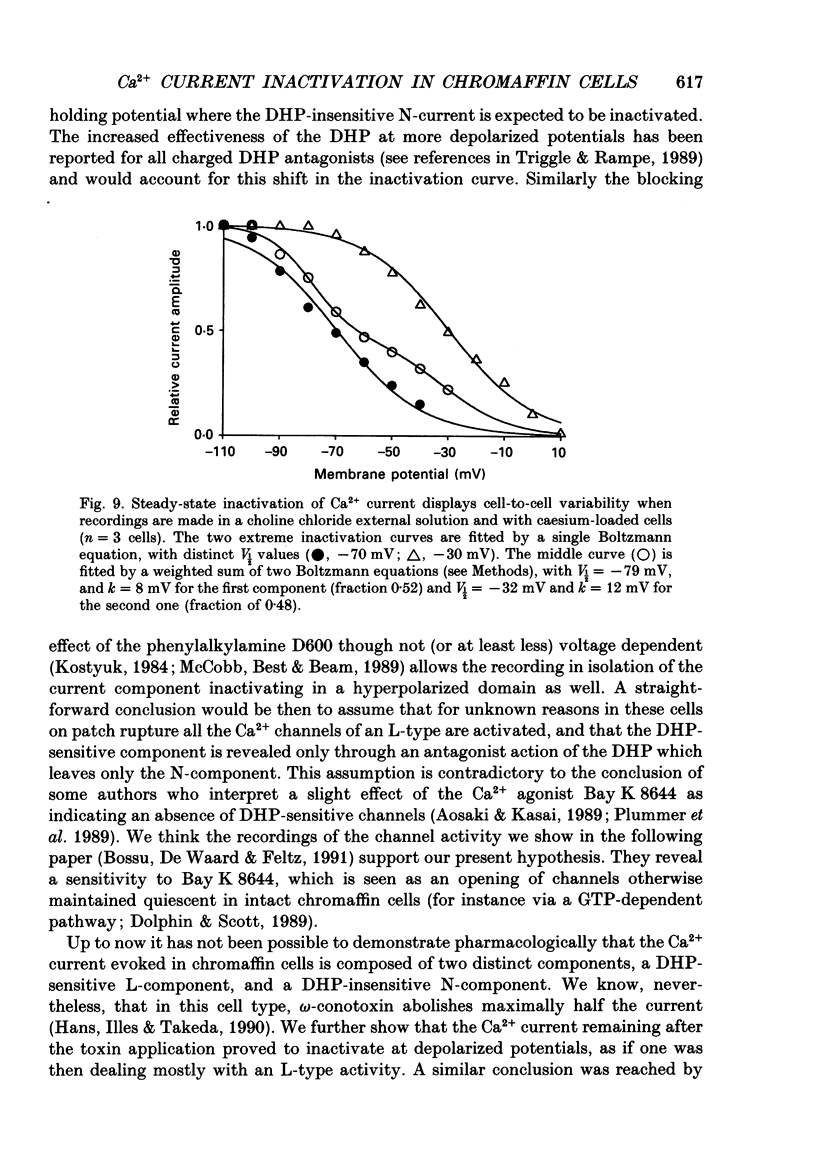
1. Two calcium currents were identified by differences in their inactivation characteristics in adult chromaffin cells maintained in short-term primary culture (3-5 days). Calcium currents were recorded by means of the whole-cell configuration using an intracellular medium highly buffered for pH and pCa. 2. Calcium current evoked from a holding potential of -90 mV inactivated along two components: an initial transient with a time constant of 250 ms followed by a plateau. 3. Steady-state inactivation followed two processes which developed at two distinct membrane potentials. One process was half-inactivated at low voltages around -55 mV and affected mainly the initial transient component. The other process, which affected mainly the sustained component of the calcium current, was half-inactivated at voltages around -10 mV. The proportions of these two processes varied greatly from cell to cell. 4. The dihydropyridine antagonists (nicardipine and nifedipine applied at 10(-5) M) and the phenylalkylamine D600 (5 x 10(-6) M) shifted the half-inactivation value towards -55 mV, indicating the suppression of the sustained component. The snail toxin, omega-conotoxin, had the opposite effect; it shifted the half-activation value towards -10 mV. 5. The calcium channel agonist Bay K 8644 (10(-5) M) either had no effect or induced only a slight increase of the response, as did its (-)-enantiomer (10(-6) M). To interpret the present results, we suggest that the L-component was maximally activated in our recording conditions. 6. In chromaffin cells, the calcium current recorded in whole-cell conditions is composed of two components with properties close to those of N- and L-type currents described in sympathetic neurons.
Full text
PDF



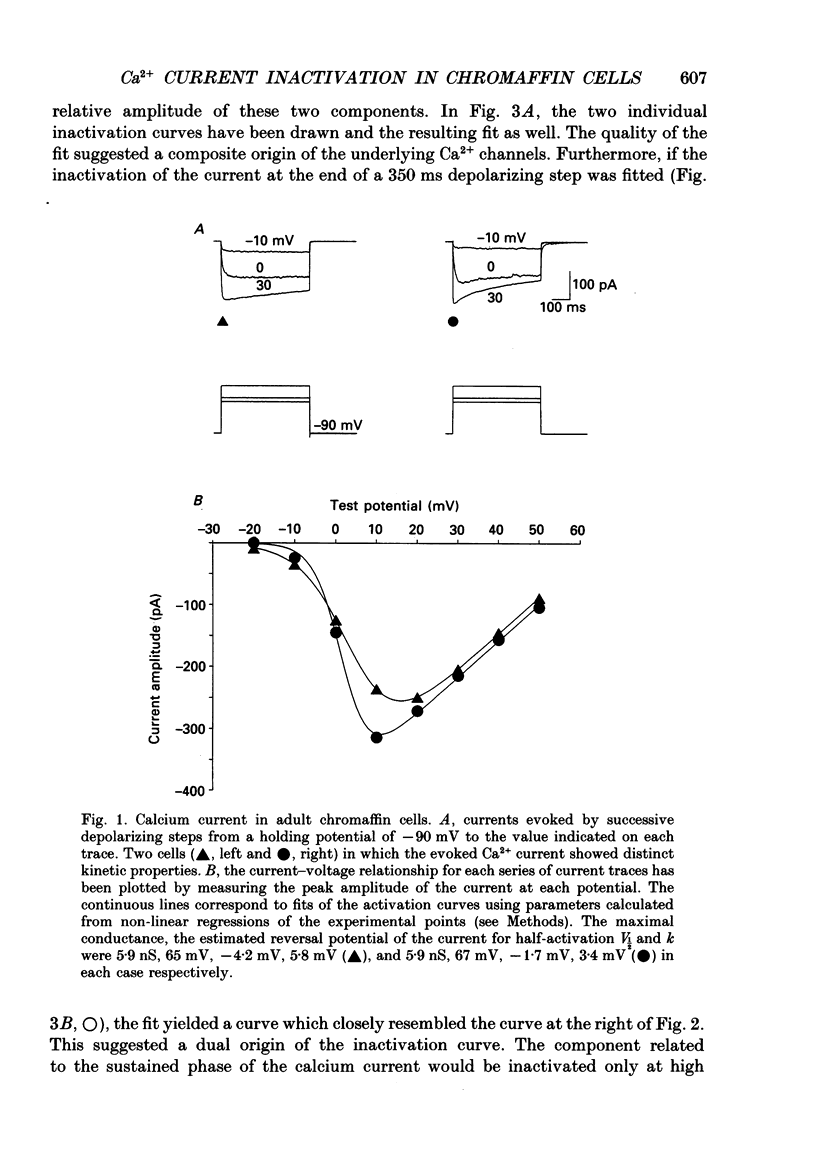
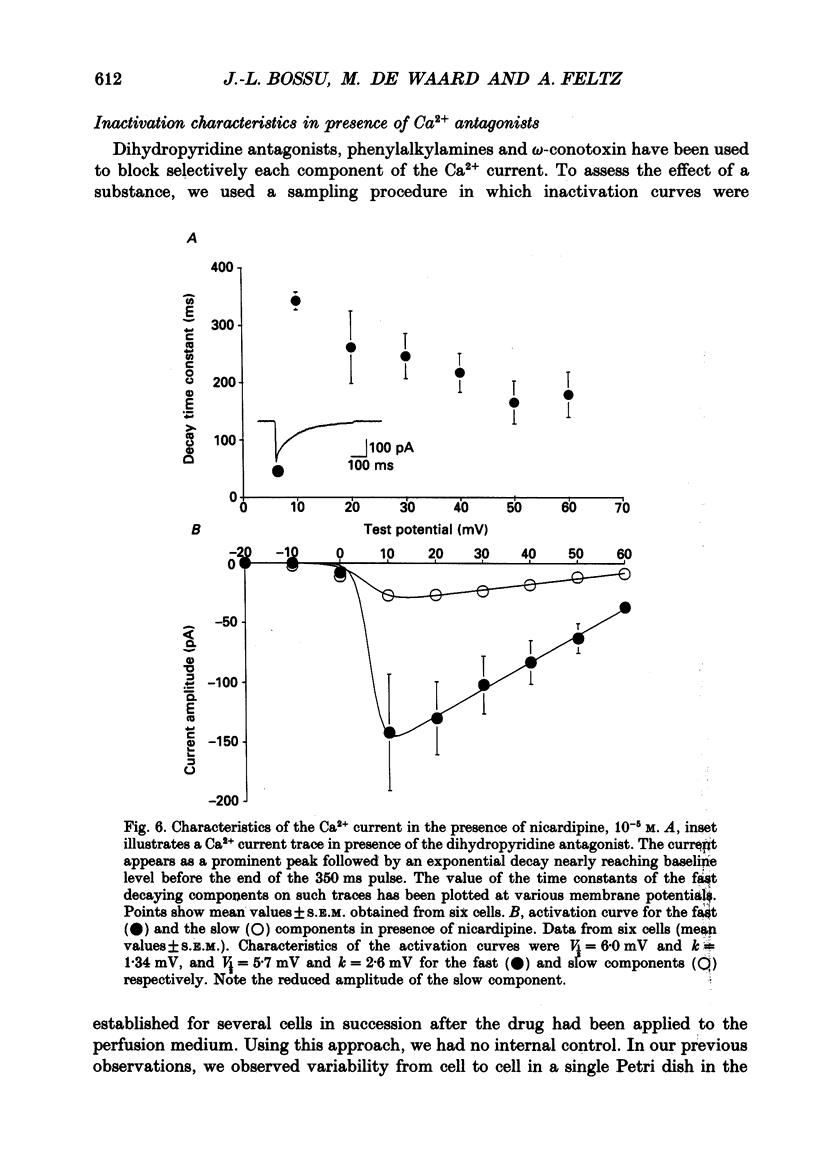
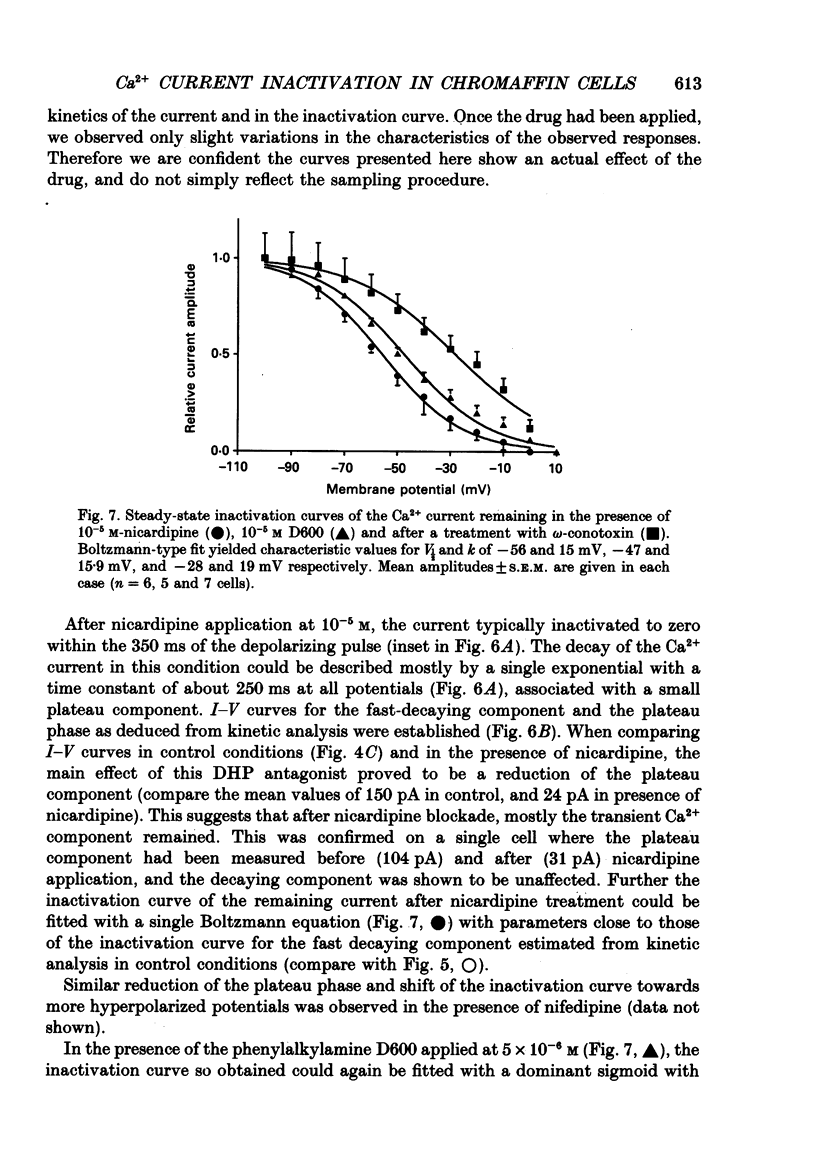





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aosaki T., Kasai H. Characterization of two kinds of high-voltage-activated Ca-channel currents in chick sensory neurons. Differential sensitivity to dihydropyridines and omega-conotoxin GVIA. Pflugers Arch. 1989 Jun;414(2):150–156. doi: 10.1007/BF00580957. [DOI] [PubMed] [Google Scholar]
- Bader M. F., Ciesielski-Treska J., Thierse D., Hesketh J. E., Aunis D. Immunocytochemical study of microtubules in chromaffin cells in culture and evidence that tubulin is not an integral protein of the chromaffin granule membrane. J Neurochem. 1981 Oct;37(4):917–933. doi: 10.1111/j.1471-4159.1981.tb04479.x. [DOI] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O. Calcium currents in the normal adult rat sympathetic neurone. J Physiol. 1989 May;412:493–512. doi: 10.1113/jphysiol.1989.sp017628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boarder M. R., Marriott D., Adams M. Stimulus secretion coupling in cultured chromaffin cells. Dependency on external sodium and on dihydropyridine-sensitive calcium channels. Biochem Pharmacol. 1987 Jan 1;36(1):163–167. doi: 10.1016/0006-2952(87)90394-7. [DOI] [PubMed] [Google Scholar]
- Bossu J. L., De Waard M., Feltz A. Two types of calcium channels are expressed in adult bovine chromaffin cells. J Physiol. 1991 Jun;437:621–634. doi: 10.1113/jphysiol.1991.sp018615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossu J. L., Rodeau J. L., Feltz A. Decay kinetics of calcium currents in rat sensory neurones: analysis at two internal free calcium concentrations. Pflugers Arch. 1989 May;414(1):89–91. doi: 10.1007/BF00585631. [DOI] [PubMed] [Google Scholar]
- Byerly L., Moody W. J. Intracellular calcium ions and calcium currents in perfused neurones of the snail, Lymnaea stagnalis. J Physiol. 1984 Jul;352:637–652. doi: 10.1113/jphysiol.1984.sp015314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claude P., Parada I. M., Gordon K. A., D'Amore P. A., Wagner J. A. Acidic fibroblast growth factor stimulates adrenal chromaffin cells to proliferate and to extend neurites, but is not a long-term survival factor. Neuron. 1988 Nov;1(9):783–790. doi: 10.1016/0896-6273(88)90126-2. [DOI] [PubMed] [Google Scholar]
- Demeneix B., Grant N. J. Alpha-melanocyte stimulating hormone promotes neurite outgrowth in chromaffin cells. FEBS Lett. 1988 Jan 4;226(2):337–342. doi: 10.1016/0014-5793(88)81450-9. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Scott R. H. Interaction between calcium channel ligands and guanine nucleotides in cultured rat sensory and sympathetic neurones. J Physiol. 1989 Jun;413:271–288. doi: 10.1113/jphysiol.1989.sp017653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe A. J., Landis S. C., Patterson P. H. Environmental influences in the development of neural crest derivatives: glucocorticoids, growth factors, and chromaffin cell plasticity. J Neurosci. 1985 Aug;5(8):2119–2142. doi: 10.1523/JNEUROSCI.05-08-02119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandía L., López M. G., Fonteríz R. I., Artalejo C. R., García A. G. Relative sensitivities of chromaffin cell calcium channels to organic and inorganic calcium antagonists. Neurosci Lett. 1987 Jun 26;77(3):333–338. doi: 10.1016/0304-3940(87)90523-4. [DOI] [PubMed] [Google Scholar]
- García A. G., Sala F., Reig J. A., Viniegra S., Frías J., Fontériz R., Gandía L. Dihydropyridine BAY-K-8644 activates chromaffin cell calcium channels. Nature. 1984 May 3;309(5963):69–71. doi: 10.1038/309069a0. [DOI] [PubMed] [Google Scholar]
- Greenberg D. A., Carpenter C. L., Cooper E. C. Stimulation of calcium uptake in PC12 cells by the dihydropyridine agonist BAY K 8644. J Neurochem. 1985 Sep;45(3):990–993. doi: 10.1111/j.1471-4159.1985.tb04095.x. [DOI] [PubMed] [Google Scholar]
- Gross R. A., Macdonald R. L. Differential actions of pentobarbitone on calcium current components of mouse sensory neurones in culture. J Physiol. 1988 Nov;405:187–203. doi: 10.1113/jphysiol.1988.sp017328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans M., Illes P., Takeda K. The blocking effects of omega-conotoxin on Ca current in bovine chromaffin cells. Neurosci Lett. 1990 Jun 22;114(1):63–68. doi: 10.1016/0304-3940(90)90429-d. [DOI] [PubMed] [Google Scholar]
- Hirning L. D., Fox A. P., McCleskey E. W., Olivera B. M., Thayer S. A., Miller R. J., Tsien R. W. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988 Jan 1;239(4835):57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- Janigro D., Maccaferri G., Meldolesi J. Calcium channels in undifferentiated PC12 rat pheochromocytoma cells. FEBS Lett. 1989 Sep 25;255(2):398–400. doi: 10.1016/0014-5793(89)81131-7. [DOI] [PubMed] [Google Scholar]
- Jones S. W., Marks T. N. Calcium currents in bullfrog sympathetic neurons. I. Activation kinetics and pharmacology. J Gen Physiol. 1989 Jul;94(1):151–167. doi: 10.1085/jgp.94.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G. Intracellular perfusion of nerve cells and its effects on membrane currents. Physiol Rev. 1984 Apr;64(2):435–454. doi: 10.1152/physrev.1984.64.2.435. [DOI] [PubMed] [Google Scholar]
- López M. G., Moro M. A., Castillo C. F., Artalejo C. R., García A. G. Variable, voltage-dependent, blocking effects of nitrendipine, verapamil, diltiazem, cinnarizine and cadmium on adrenomedullary secretion. Br J Pharmacol. 1989 Mar;96(3):725–731. doi: 10.1111/j.1476-5381.1989.tb11874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti C., Carbone E., Lux H. D. Effects of dopamine and noradrenaline on Ca channels of cultured sensory and sympathetic neurons of chick. Pflugers Arch. 1986 Feb;406(2):104–111. doi: 10.1007/BF00586670. [DOI] [PubMed] [Google Scholar]
- Marqueze B., Martin-Moutot N., Levêque C., Couraud F. Characterization of the omega-conotoxin-binding molecule in rat brain synaptosomes and cultured neurons. Mol Pharmacol. 1988 Aug;34(2):87–90. [PubMed] [Google Scholar]
- McCleskey E. W., Fox A. P., Feldman D. H., Cruz L. J., Olivera B. M., Tsien R. W., Yoshikami D. Omega-conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4327–4331. doi: 10.1073/pnas.84.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCobb D. P., Best P. M., Beam K. G. Development alters the expression of calcium currents in chick limb motoneurons. Neuron. 1989 Jun;2(6):1633–1643. doi: 10.1016/0896-6273(89)90052-4. [DOI] [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989 Jul 20;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Owen P. J., Marriott D. B., Boarder M. R. Evidence for a dihydropyridine-sensitive and conotoxin-insensitive release of noradrenaline and uptake of calcium in adrenal chromaffin cells. Br J Pharmacol. 1989 May;97(1):133–138. doi: 10.1111/j.1476-5381.1989.tb11933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher E., Pandiella A., Clementi F. Omega-conotoxin binding and effects on calcium channel function in human neuroblastoma and rat pheochromocytoma cell lines. FEBS Lett. 1988 Aug 1;235(1-2):178–182. doi: 10.1016/0014-5793(88)81258-4. [DOI] [PubMed] [Google Scholar]
- Streit J., Lux H. D. Voltage dependent calcium currents in PC12 growth cones and cells during NGF-induced cell growth. Pflugers Arch. 1987 May;408(6):634–641. doi: 10.1007/BF00581167. [DOI] [PubMed] [Google Scholar]
- Swandulla D., Armstrong C. M. Fast-deactivating calcium channels in chick sensory neurons. J Gen Physiol. 1988 Aug;92(2):197–218. doi: 10.1085/jgp.92.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Tsukui H., Hatanaka H. Neuronal differentiation of Ca2+ channel by nerve growth factor. Brain Res. 1985 Aug 26;341(2):381–384. doi: 10.1016/0006-8993(85)91079-0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Triggle D. J., Rampe D. 1,4-Dihydropyridine activators and antagonists: structural and functional distinctions. Trends Pharmacol Sci. 1989 Dec;10(12):507–511. doi: 10.1016/0165-6147(89)90051-5. [DOI] [PubMed] [Google Scholar]
- Unsicker K., Krisch B., Otten U., Thoenen H. Nerve growth factor-induced fiber outgrowth from isolated rat adrenal chromaffin cells: impairment by glucocorticoids. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3498–3502. doi: 10.1073/pnas.75.7.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]


