FIG. 3.
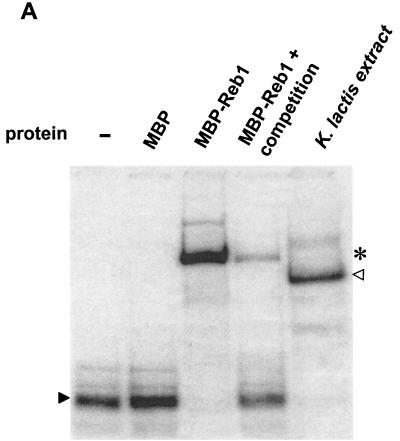
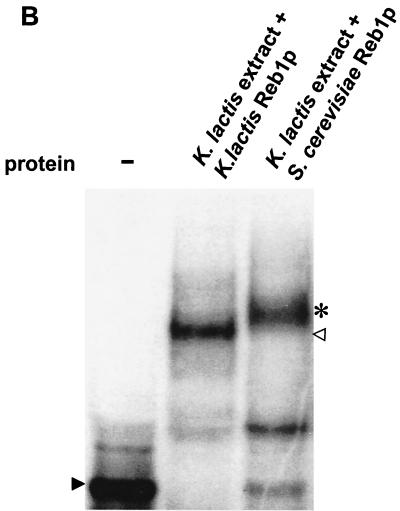
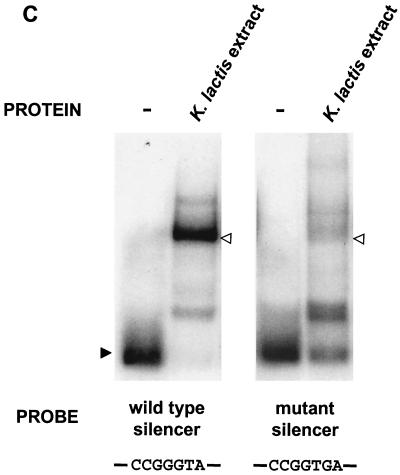
Reb1p binds to the minimal silencer. (A) Recombinant MBP-Reb1p bound to the minimal silencer. An electrophoretic mobility shift assay (EMSA) of labeled 102-bp minimal silencer incubated without protein (−), with MBP alone, MBP-Reb1p, and a K. lactis protein extract was performed. Additionally, wild-type unlabeled silencer at a 30-fold molar excess was incubated together with MBP-Reb1p to compete for labeled silencer binding. A solid arrowhead indicates unbound minimal silencer, an open arrowhead indicates the Reb1p mobility shift, and an asterisk indicates the MBP-Reb1p shift. (B) Reb1p was the major protein that bound to the silencer in K. lactis protein extracts. Protein extracts prepared from reb1::hisG strain SAY206, containing plasmids carrying either the K. lactis REB1 gene or the S. cerevisiae REB1 gene, were tested in an EMSA for the ability to bind the minimal silencer. Since the K. lactis and S. cerevisiae Reb1p proteins are of different sizes, the S. cerevisiae Reb1p mobility shift (asterisk) migrated slower than the K. lactis Reb1p mobility shift (open triangle). The unbound silencer is indicated by the solid arrowhead. (C) Inefficient binding of a silencer containing point mutations in the Reb1p binding site. EMSA with either a labeled wild-type silencer fragment or a labeled silencer containing two point mutations in the Reb1p binding site (Reb1− in Fig. 1A) was performed. A K. lactis protein extract was incubated either with wild-type silencer or the Reb1− silencer. −, no protein added to the reaction mixture. The free probe is indicated by a solid arrowhead, and the shifted probe is indicated by an open arrowhead.