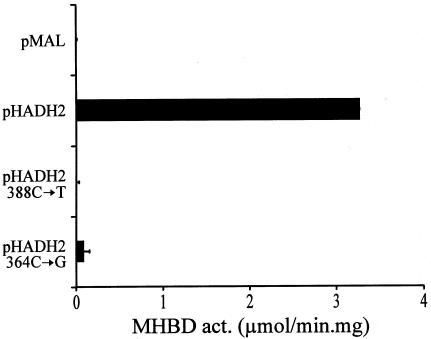
Figure 5.
Heterologous expression of human MHBD in E. coli. The complete ORF of HADH2 from a control subject and from patients with MHBD deficiency were amplified and ligated into the bacterial expression vector pMAL-C2X, as described. Each ORF was sequenced to exclude sequence errors introduced during PCR. Transformed bacteria (INVα) were grown in 20-ml LB medium supplemented with 100 μg/ml ampicillin to an OD600 of ∼0.5, and IPTG was added to a final concentration of 1 mM to induce protein expression. After 4 h at 37°C, cells were pelleted and resuspended in 1 ml 10 mM sodium phosphate buffer pH 7.4, containing 140 mM NaCl, 0.1% (wt/vol) Triton X-100 and protease inhibitors (1 tablet Completemini [Boehringer Mannheim] in 10-ml solution). Lysis was achieved by sonication at 9 W for 10 s. The bacterial lysate was centrifuged for 10 min at 14,000×gav, and the pellet was discarded. The supernatant was used for protein measurement and MHBD activity, as described. Expression levels of the fusion protein in the bacterial extracts were equal, on the basis of immunoblot analysis (data not shown). pMAL = expression with empty pMAL-c2x vector; pHADH2 = expression with vector containing the wild-type human MHBD; pHADH2 388C→T = expression of human MHBD mutant with R130C; pHADH2 364C→G = expression of human MHBD mutant with L122V.