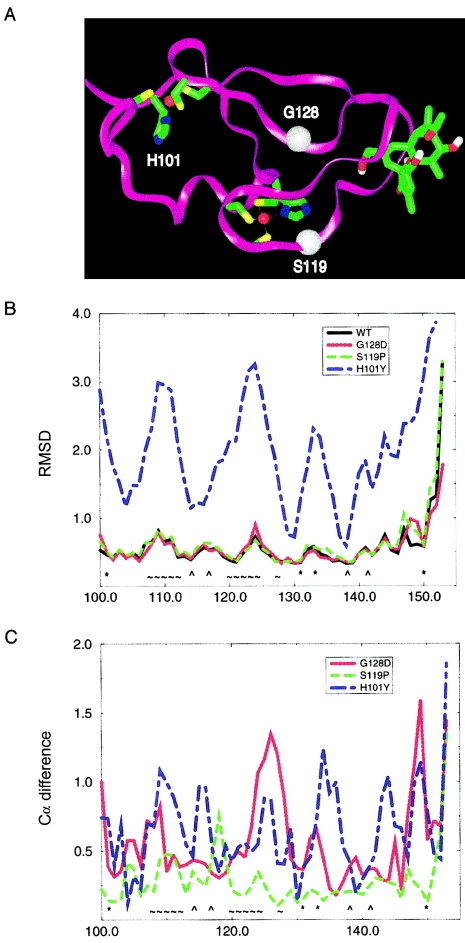
Figure 4.
MD studies of wild-type and mutant Cys2 regions of PKCγ. A,Three-dimensional structure of the Cys2 region of wild-type PKCγ with phorbol acetate bound. H101 is a ligand in the first zinc binding site; zinc is shown in red. S119 is close to the second zinc binding site, as well as the phorbol acetate binding site. G128 is also in the vicinity of the phorbol acetate binding site; Cα positions of S119 and G128 are indicated by white spheres. B, Fluctuations of protein backbone coordinates per residue, expressed as root-mean-square deviation (RMSD) (in Å) for wild type and three mutants. Note the instability of the H101Y mutant in comparison to wild type and the two other mutants. C, Differences in average structures, calculated as distance between Cα positions (in Å) per residue between wild type and each of the three mutants. Note the global shift of the structure in the H101Y mutant and the shifts in the phorbol ester binding site in the G128D mutant. WT = wild type; * = first zinc binding site; ⁁ = second zinc binding site; ∼ = phorbol binding site.