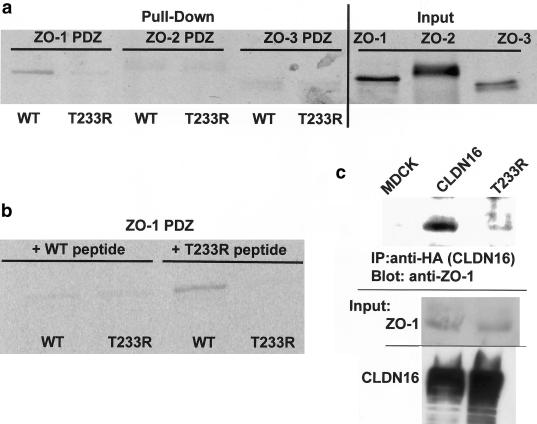
Figure 3.
Binding of wild-type and mutant CLDN16 to ZO-1. a, Wild-type CLDN16, but not the T233R mutant, binds to the ZO-1 but not the ZO-2 and ZO-3 PDZ domains. In vitro translated and radioactively labeled ZO-1, ZO-2, or ZO-3 PDZ domains were incubated with peptides corresponding to the C-terminus of wild-type or T233R mutant CLDN16 coupled to beads. Protein bound to the beads (“Pull-Down”) was analyzed by SDS-PAGE and autoradiography, and an aliquot (5%) of the in vitro translated material was directly analyzed to confirm that similar amounts of the in vitro translated proteins were added to the binding reaction (“Input”). b, Preincubation with wild-type, but not T233R mutant, peptides competes with ZO-1 PDZ binding to beads. In vitro translated and radioactively labeled ZO-1 PDZ domains were pre-incubated with soluble wild-type or mutant CLDN16 peptides prior to the incubation with the peptide-coupled beads. c, Wild-type CLDN16, but not the T233R mutant, coprecipitates with ZO-1. Control cells (MDCK) or cells stably expressing wild-type (CLDN16) or mutant (T233R) CLDN16 were lysed, and equal amounts of total protein were used to immunoprecipitate CLDN16. Precipitates were blotted with anti–ZO-1 antibody to detect ZO-1 bound to CLDN16. In the reverse experiment, wild-type, but not mutant CLDN16, which is also associated with anti–ZO-1, precipitates (data not shown). Aliquots of the cell lysate were blotted directly to confirm that the cells expressed similar amounts of ZO-1, as well as wild-type and mutant CLDN16 (“Input”). WT = wild type.