Abstract
Background
Streptozocin (STZ) administration with or without other cytotoxic drugs remains a crucial chemotherapy for patients with advanced pancreatic neuroendocrine neoplasms (Pan-NENs). However, the therapeutic effects of combination treatment with weekly STZ and oral S-1 therapy (STS1) remain unknown. Therefore, the aim of this study was to evaluate the safety and clinical feasibility of STS1.
Methods
Twenty of 243 Pan-NEN patients were included in this retrospective study, all of whom had received STS1 for unresectable or distant metastatic diseases from November 2015 to January 2019. The maximum tumor shrinkage rate, time course of the tumor shrinkage rate, prognosis (progression-free survival and overall survival), and adverse events were evaluated.
Results
The median age of the patients was 61.5 years and the median tumor size was 35 mm. The number of NET-G1, NET-G2, NET-G3, and NEC-G3 patients was 3, 13, 3, and 1, respectively. The median Ki-67 index and mitoses were 10.2% and 2/10 high-power fields, respectively. The overall objective response rate and disease control rate were 30% and 90%, respectively. The median maximum tumor reduction rate was 19%. The Ki-67 index and tumor size did not influence the tumor shrinkage rate. Progression-free survival after STS1 treatment was 19 months with no significant difference between NET-G1/G2 and NET-G3/NEC-G3 patients (p = 0.4). There was one case each of grade 3/4 toxicity, including general fatigue, hyperglycemia, and renal dysfunction. No serious myelosuppressive events are manifested.
Conclusions
STS1 treatment is an effective and safe therapeutic option for patients with advanced Pan-NEN.
Electronic supplementary material
The online version of this article (10.1007/s00432-019-03109-5) contains supplementary material, which is available to authorized users.
Keywords: Pancreatic neuroendocrine neoplasms, Streptozocin, Multidisciplinary treatment
Introduction
Pancreatic neuroendocrine neoplasms (Pan-NENs) are tumors arising from pancreatic endocrine cells (Halfdanarson et al. 2008) and are generally considered to be clinically rare; however, the incidence of these tumors has recently been increasing (Hauso et al. 2008; Ito et al. 2010; Yao et al. 2008). Disease outcomes are certainly dependent on tumor grade, classified as NET-G1, G2, G3, and NEC-G3 based on the updated modification of the 2017 World Health Organization (WHO) classification.
Metastases to distant organs are often present at the time of diagnosis of Pan-NENs (Kouvaraki et al. 2004; Panzuto et al. 2011). In particular, liver metastases are of relevance to the disease prognosis (Frilling et al. 2014). Surgical resection is a curative treatment for patients with Pan-NENs, however; systemic chemotherapy is administered for cases that are surgically unresectable. Various types of treatment options for Pan-NENs have been established and the decision for systemic therapy is based on biological factors such as the tumor burden, grade, or growth rate (Pavel et al. 2016).
Recently, molecular target drugs have played a central role in the treatment of unresectable Pan-NENs. In fact, sunitinib and everolimus have been approved for standard therapy mainly in progressive NET-G1 and G2 Pan-NENs (Pavel et al. 2016; Raymond et al. 2011; Yao et al. 2011). Streptozocin (STZ) based chemotherapy is also regarded as a therapeutic agent for advanced locoregional or distant metastatic Pan-NENs (Pavel et al. 2016). STZ has cytotoxic efficacy to inhibit DNA synthesis leading to cancer cell death (Bolzan and Bianchi 2002). In general, the clinical efficacy of STZ has been reported according to a daily-based regimen in combination with 5-fluorouracil (5-FU) or doxorubicin (Antonodimitrakis et al. 2016; Dilz et al. 2015; Kouvaraki et al. 2004; Krug et al. 2015; Moertel et al. 1980, 1992); however, the clinical effectiveness of a weekly based regimen of STZ, especially when combined with 5-FU, remains uncertain.
S-1 is an oral fluoropyrimidine compound comprising tegafur, gimeracil, and oteracil potassium. This combination is designed to enhance the antitumor effect and reduce the gastrointestinal toxicity of 5-FU (Shirasaka et al. 1996). The safety and efficacy of S-1-based combination regimens have been confirmed in the treatment of several types of cancers such as gastric and colorectal cancers (Boku et al. 2009; Koizumi et al. 2008; Muro et al. 2010; Yamada et al. 2013). S-1 has been recognized to demonstrate similar efficacy with acceptable clinical complications compared to therapeutic regimens involving the intravenous administration of 5-FU in an advanced disease setting.
In this study, we investigated the clinical efficacy and safety of weekly STZ administration in combination with S-1 treatment for patients with advanced Pan-NENs.
Methods
Patient and methods
This retrospective study included patients who were histologically diagnosed with Pan-NENs and treated with a weekly regimen of intravenous STZ and oral administration of S-1 (STS1 treatment) at Tokyo Medical and Dental University. After at least 2 months of treatment, the patients underwent computed tomography (CT) or magnetic resonance imaging (MRI) to evaluate the efficacy of STS1 treatment by comparing image findings before and after the treatment. A total of 20 patients were eligible for inclusion in this study who were treated between November 2017 and December 2018.
The tumors were graded according to the 2017 WHO classification. All patients were examined by contrast CT or contrast MRI every 2–3 months. The therapeutic response of STS1 treatment was evaluated by the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1). Adverse effects were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE version 5.0).
All procedures were approved by the Human Research Ethics Committee of Tokyo Medical and Dental University (Approval ID: 1080). Informed consent was documented and obtained from each patient.
STZ-based regimen (STS1 treatment)
The weekly STZ regimen was administered intravenously at a dose of 1000 mg/m2 per week. The combination STS1 treatment comprised of weekly STZ and S-1 (tegafur/gimeracil/oteracil) orally administered at a dose of 100 mg per individual every other day from day 1 (Supplemental Figure S1).
Statistical analysis
Progression-free survival (PFS) was defined as the period from the start of treatment to the appearance of progressive disease or death. Survival curves were constructed by the Kaplan–Meier method and compared with the log-rank test as necessary. A p value < 0.05 was considered statistically significant. Figures were generated using GraphPad Prism 7 software (GraphPad Software Inc., San Diego, CA, USA). Statistical analyses were performed with SPSS version 24.0 (SPSS, Chicago, IL, USA).
Results
The baseline characteristics of the patients included in this study are shown in Table 1. The median age of this cohort was 61.5 years. The patients were categorized according to tumor grade, including three cases of NET-G1 (15%), 13 cases of NET-G2 (65%), three cases of NET-G3 (15%), and 1 case of NEC-G3 (5%). The median Ki-67 percentage (a proliferation marker) was 10.2%. Eligibility for unresectable lesions comprised distant liver metastases (90%, 18/ 20 cases) and locally advanced tumors (10%, 2/ 20 cases). Liver metastases were also divided into synchronous (61.1%, 11/18 cases of liver metastasis) and metachronous as metastatic relapse after surgery (38.9%, 7/18 cases of liver metastasis).
Table 1.
Basement characteristics of STS1 treatment
| Characteristics | Patients n = 20 |
|---|---|
| Clinical | |
| Age, median (range) | 61.5 (30–76) |
| Sex, male/female | 12/8 |
| Prior surgery, n (%) | 12 (60) |
| Posterior surgery, n (%) | 2(10) |
| Prior systemic chemotherapy, n (%) | 19 (95) |
| Tumor | |
| Location | |
| Head | 9 |
| Body/tail | 11 |
| Tumor size, mm, median (range) | 35.0 (8.2–172.4) |
| Ki-67 index, %, median (range) | 10.2 (1.4–80) |
| Mitosis, per 10 HPF, median (range) | 2 (0–60) |
| Liver metastasis, n (%) | 18 (90) |
| Chromogranin A positive, n (%) | 17 (85) |
| Synaptophysin positive, n (%) | 18 (90) |
| CD56 positive, n (%) | 15 (75) |
| Tumor functionality, n (%) | |
| Non-functioning | 19 (95) |
| Gastrinoma | 1(5) |
| Tumor grade | |
| Gl | 3 |
| G2 | 13 |
| NET-G3 | 3 |
| NEC-G3 | 1 |
| STZ/S-1 treatment | |
| Month on treatment, median (range) | 6.5 (3–23.4) |
| Treatment reduction, n (%) | 6 (30) |
Nineteen patients (95%) had received systemic chemotherapy before STS1 treatment and the treatment was started as second-line or later chemotherapy. Most of the patients had received sunitinib treatment (90%, 18/20 cases) before STS1 treatment was initiated per the protocol of our institute. The median duration of STS1 treatment was 6.5 months. Treatment dose reduction was performed due to toxicity in six patients and 13 patients (65%) were continuing the present treatment during this analysis. Among seven patients who withdrew from treatment, surgical resection was performed in two cases, including one case of stable disease (SD) and one case of progressive disease (PD).
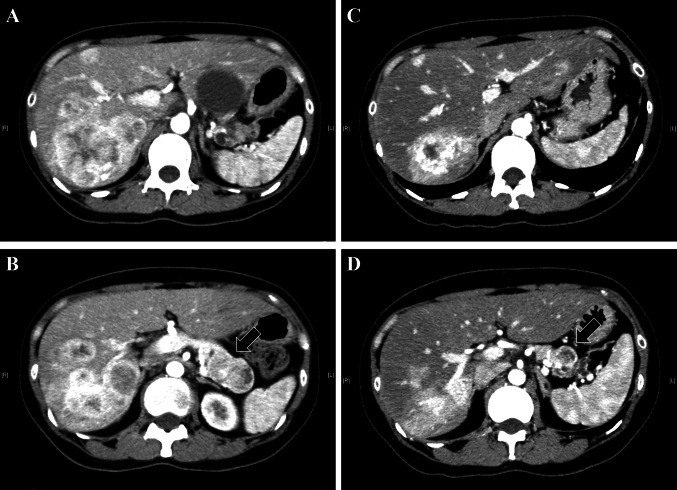
Figure 1a shows the best tumor response rate throughout the administration period. Six patients (30%) achieved partial remission (PR), including a mixed response in one case and no patient achieved complete remission (CR). Twelve patients (60%) showed SD at the best response. Notably, STS1 treatment resulted in a disease control rate (DCR: PR + SD) of 90%. The median maximum tumor reduction rate after STS1 treatment was 19% in these patients. With respect to the characteristic feature of tumor shrinkage by STS1 treatment, once the treatment was started and the tumor was well controlled by the treatment, the therapeutic efficacy seemed to be maintained (Fig. 1b). In fact, long-term persistence of disease control over 20 months was confirmed in five cases (40%, four with a PR and one with SD). Figure 2 shows representative imaging findings of contrast CT examination with a remarkable tumor response before and after 8 months of STS1 treatment (corresponding to case no. 12 in Supplementary Table S1). The primary lesion (NET-G2) was in the body of the pancreas. Numerous metastatic foci were noted in the bilateral lobes of the liver and the maximum tumor diameter located in the right inferior lobe was 59 mm. This patient had a high tumor burden; however, the Ki-67 index was only 3.4%.
Fig. 1.
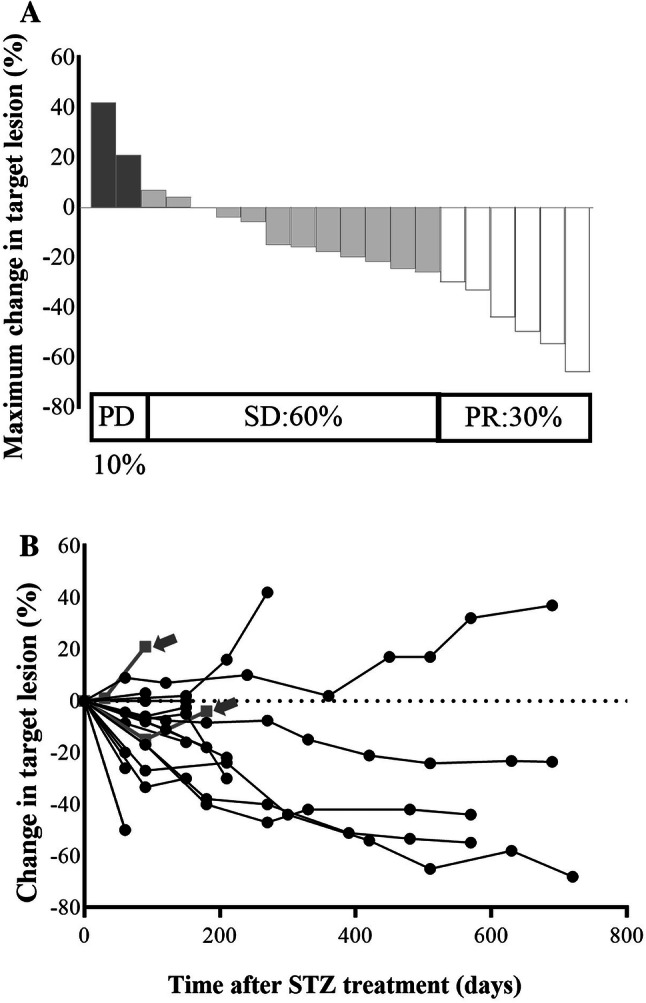
Tumor shrinkage rates after STS1 treatment. a Maximum shrinkage rate of the target lesion evaluated by RECIST. PD progressive disease, SD stable disease, PR partial response. Maximum shrinkage rate (%) = [(sum of tumor diameters with a maximum reduction/increase – baseline diameters)/baseline diameters] × 100 for each patient. b Time course of size in the target lesion in each patient. Notably, once the treatment was started and the tumor was controlled by the treatment, the therapeutic efficacy seemed to be maintained. Gray narrow represents surgical resected cases following STS1 treatment
Fig. 2.
Representative imaging findings of contrast CT examination in a patient with multiple liver metastases. Black arrow, primary lesion located in the body of the pancreas. a, b Liver metastases (a) and primary lesion (b) before STS1 treatment as baseline levels. c, d Remarkable response of liver metastases (c) and the primary lesion (d) after 8 months of STS1 treatment
The median PFS after STS1 treatment was 19 months, as analyzed by the Kaplan–Meier method [95% confidence interval (CI) 13.3–26.1 months; Fig. 3a]. The median overall survival after STS1 treatment was not reached in this study (95% CI 23.1–32.0 months); however, the 2-year survival rate was 82.1%, as analyzed by the Kaplan–Meier method (Fig. 3b). Five patients underwent short-term treatment (under 9 months) due to disease progression (n = 4), toxicity (fatigue, n = 1), and SD as the best response (n = 1). Thereafter, two of these patients underwent surgical resection (one case of PD and SD each).
Fig. 3.
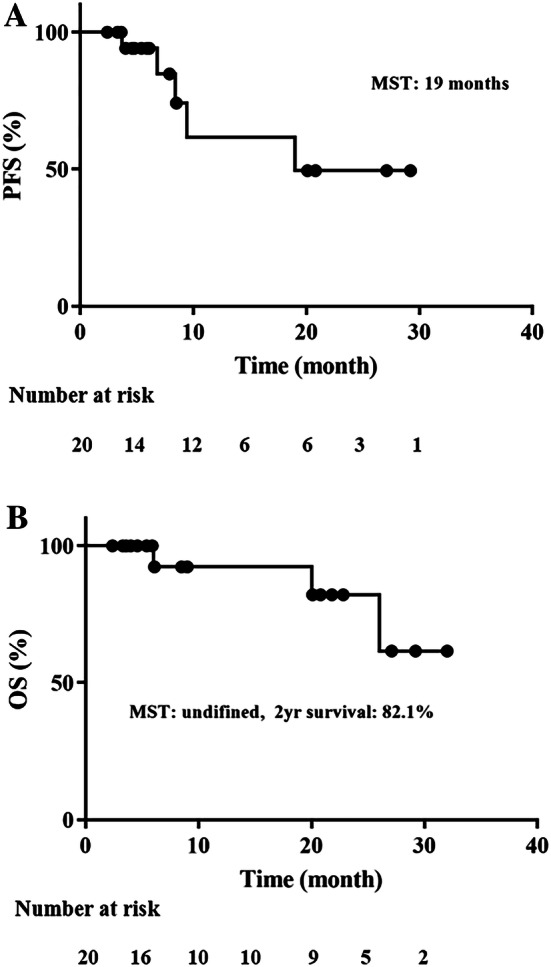
Progression-free survival (a) and overall survival (b) rates from the starting day of STS1 treatment in patients with pancreatic neuroendocrine neoplasms
Tumor progression was better controlled by the treatment in NET-G1/G2 patients than in NET-G3/NEC-G3 patients and there was no significant difference in PFS between the two groups (Fig. 4). Moreover, the Ki-67 index and tumor size did not emerge as significant variables related to the clinical efficacy of STS1 treatment (Supplementary Table S2). These data suggested that STS1 treatment was effective regardless of the tumor grade or size.
Fig. 4.
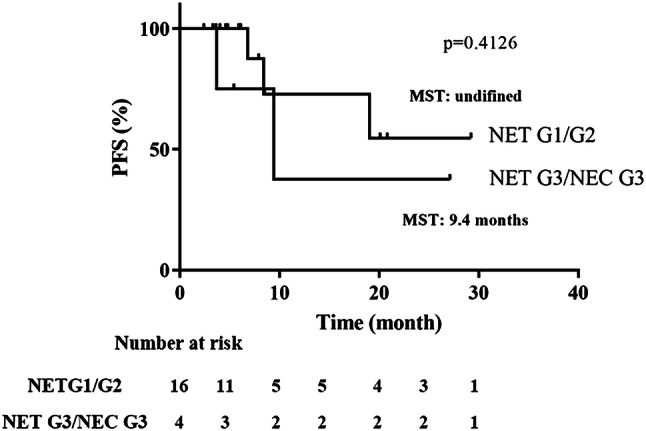
Progression-free survival rates in NET-G1/G2 and NET-G3/NEC-G3 tumors. There was no significant difference between the two groups
In terms of adverse effects, events were observed in 16 patients (80%) (Table 2). The incidence of events higher than grade 3 occurred in 3 of 20 patients (15%). Renal dysfunction was the most frequent event, which occurred in ten patients (50%).
Table 2.
Clinical side effects of STS1 treatment
| All grades (n, %) | Grade 3/4 (n, %) | |
|---|---|---|
| Fatigue | 7 (35%) | 1 (5%) |
| Gastrointestinal | ||
| Anorexia | 2 (10%) | |
| Constipation | 9 (45%) | |
| Joint pain | 2 (10%) | |
| Nasal bleeding | 2 (10%) | |
| Increased blood glucose | 3 (15%) | 1 (5%) |
| Hematologic | ||
| Thromobocytopenia | 4 (20%) | |
| Neutropenia | 1 (5%) | |
| Anemia | 1 (5%) | |
| Renal | ||
| Renal dysfunction | 10 (50%) | 1 (5%) |
| Proteinuria | 3 (15%) |
Discussion
Cytotoxic systemic chemotherapy can be indicated as one of the treatment options for patients with unresectable or metastatic diseases. STZ has been established as an effective antitumor treatment for advanced Pan-NENs. However, its indication and treatment strategy have not yet been clearly determined (Pavel et al. 2016). In general, STZ treatment is carried out as monotherapy or combination therapy with 5-FU or doxorubicin (Antonodimitrakis et al. 2016; Dilz et al. 2015; Kouvaraki et al. 2004; Krug et al. 2015; Moertel et al. 1980, 1992; Pavel et al. 2016). A previous study also revealed that STZ-based combination therapy is preferable to STZ monotherapy for the treatment of Pan-NENs (Moertel et al. 1980). In addition, the clinical use of doxorubicin requires attention to the risk of cardiotoxicity (Pavel et al. 2016). Therefore, there have been many studies focusing on the effects of STZ and 5-FU combination therapy.
Here, we provide the first report of the effects of weekly STZ administration in combination with 5-FU replacing oral S-1 treatment for patients with advanced Pan-NENs. Weekly STZ in combination with S-1 (STS1) treatment showed an objective response rate (ORR) of 30%, a DCR of 90%, and a median PFS of 19 months. In our cohort, most of the patients presented distant metastasis of the liver (90%) and a tumor grade of NET-G2 (65%). The background characteristics of our patients, including tumor grade, are similar to those reported previously (Antonodimitrakis et al. 2016; Dilz et al. 2015; Krug et al. 2015; Shibuya et al. 2018; Turner et al. 2010). According to a previous multicenter study on STZ treatment, in which our institute also participated, STZ treatment as monotherapy and with a small portion as combination therapy resulted in an ORR of 21.8%, DCR of 69.1%, and median PFS of 9.8 months (Shibuya et al. 2018). S-1 oral administration can be considered to provide an additional antitumor effect and synergistically enhances the cytotoxic property of STZ treatment. Consistent with previous studies concerning 5-FU combination (PFS 14–23 months, ORR 28–63%) (Antonodimitrakis et al. 2016; Frilling and Clift 2015; Krug et al. 2015; Moertel et al. 1980, 1992), our result demonstrated a favorable therapeutic efficacy. These data indicate that STS1 treatment is equally effective as combination STZ/5-FU treatment in the advanced disease settings of Pan-NENs.
In general, STZ combination therapy with 5-FU has previously been evaluated with a daily regimen (Antonodimitrakis et al. 2016; Dilz et al. 2015; Kouvaraki et al. 2004; Krug et al. 2015; Moertel et al. 1980, 1992). In contrast, STS1 treatment in the present study was designed as ambulatory cancer chemotherapy. Thus, this regimen can contribute a clinical benefit in the management of patients with advanced Pan-NENs.
STZ plays an important role in the treatment of advanced Pan-NENs with a high Ki-67 index, but can also be performed in patients with a low Ki-67 index and G1/G2 Pan-NENs as necessary. There is evidence to suggest that the tumor Ki-67 index may be predictive for the tumor response to STZ treatment (Krug et al. 2015; Turner et al. 2010). Recently, it was also reported that a Ki-67 index over 5% could be a predictive marker of the ORR for STZ treatment (Shibuya et al. 2018). However, there is still no established consensus of the Ki-67 cut-off value of an STZ-based regimen (Pavel et al. 2016). Evidence of a predictive marker for an STZ-based regimen is also limited. In this study, the combination treatment was also carried out for patients with advanced Pan-NENs with a Ki-67 index below 5%. As shown in Fig. 4 and Supplementary Table S2, there was no significant difference in the therapeutic response regarding the Ki-67 index or tumor size, indicating that a weekly regimen of STZ in combination with oral S-1 administration is effective against advanced Pan-NENs regardless of the tumor burden and tumor grade.
In the present study, 95% of the patients received STS1 treatment after tumor progression under previous treatment with sunitinib. This evidence convincingly proves the clinical utility of STS1 treatment even after second-line chemotherapy, which can expand the treatment options for advanced Pan-NENs. In fact, the significant usefulness of sunitinib for the treatment of NET-G3 was already reported by our group (Mizuno et al. 2018). However, there is no clear consensus as to whether a cytotoxic chemotherapy regimen should be used. Thus, a treatment strategy with sequential administration of STS1 following sunitinib might play a crucial role in contributing to a better prognosis for patients with advanced Pan-NENs with high proliferative potential, such as NET-G3.
In terms of adverse effects, serious events (defined as grades 3 and 4 by the CTCAE) were demonstrated, including one case each of fatigue, increased blood glucose, or renal dysfunction. The incidence of grades 3 and 4 adverse events remains lower than that of other reports with combination therapy (Kouvaraki et al. 2004; Krug et al. 2015; Turner et al. 2010) and other therapies (Raymond et al. 2011; Yao et al. 2011). Moreover, there were no severe myelosuppressive events in our patients such as leukopenia, anemia, and thrombocytopenia. Renal dysfunction is considered as one of the highly relevant toxicities for STZ treatment (Moertel et al. 1980, 1992). The incidence of renal dysfunction was comparable to that of other reports (22–65.5% at any stage) (Antonodimitrakis et al. 2016; Dilz et al. 2015; Krug et al. 2015), and two patients reduced the dose of S-1 or STZ due to renal dysfunction; however, a sustained durable response was confirmed even after dose reduction. These results support that STS1 treatment has an acceptable safety profile.
There are some experimental limitations of this study worth mentioning. This was an observational retrospective study predominantly involving treatment with Asian patients and was performed over a somewhat short observational period. In addition, given the limited sample size, further studies with more patients are required to analyze the therapeutic efficacy and determine its indication.
In conclusion, this study demonstrated that STS1 treatment results in reliable and feasible outcomes with a favorable adverse effect profile. This regimen could be promising in the treatment of patients with unresectable advanced Pan-NENs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by Grant-in-Aid for Scientific Research (C); Grant Number 19K09041.
Compliance with ethical standards
Conflict of interest
None of the authors have personal conflicts of interest associated with the publication of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Antonodimitrakis PC, Sundin A, Wassberg C, Granberg D, Skogseid B, Eriksson B (2016) Streptozocin and 5-fluorouracil for the treatment of pancreatic neuroendocrine tumors: efficacy, prognostic factors and toxicity. Neuroendocrinology 103:345–353. 10.1159/000439086 [DOI] [PubMed] [Google Scholar]
- Boku N et al (2009) Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study Lancet Oncol 10:1063–1069. 10.1016/S1470-2045(09)70259-1 [DOI] [PubMed] [Google Scholar]
- Bolzan AD, Bianchi MS (2002) Genotoxicity of streptozotocin. Mutat Res 512:121–134 [DOI] [PubMed] [Google Scholar]
- Dilz LM et al (2015) Streptozocin/5-fluorouracil chemotherapy is associated with durable response in patients with advanced pancreatic neuroendocrine tumours Eur J Cancer 51:1253–1262. 10.1016/j.ejca.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Frilling A et al (2014) Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol 15:e8–21. 10.1016/S1470-2045(13)70362-0 [DOI] [PubMed] [Google Scholar]
- Frilling A, Clift AK (2015) Therapeutic strategies for neuroendocrine liver metastases. Cancer 121:1172–1186. 10.1002/cncr.28760 [DOI] [PubMed] [Google Scholar]
- Halfdanarson TR, Rabe KG, Rubin J, Petersen GM (2008) Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol 19:1727–1733. 10.1093/annonc/mdn351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauso O, Gustafsson BI, Kidd M, Waldum HL, Drozdov I, Chan AK et al (2008) Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer 113:2655–2664. 10.1002/cncr.23883 [DOI] [PubMed] [Google Scholar]
- Ito T et al (2010) Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol 45:234–243. 10.1007/s00535-009-0194-8 [DOI] [PubMed] [Google Scholar]
- Koizumi W et al. (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9:215–221. 10.1016/S1470-2045(08)70035-4 [DOI] [PubMed] [Google Scholar]
- Kouvaraki MA, Ajani JA, Hoff P, Wolff R, Evans DB, Lozano R, Yao JC (2004) Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol 22:4762–4771. 10.1200/JCO.2004.04.024 [DOI] [PubMed] [Google Scholar]
- Krug S et al (2015) Streptozocin-based chemotherapy in patients with advanced neuroendocrine neoplasms-predictive and prognostic markers for treatment stratification. PLoS One 10:e0143822. 10.1371/journal.pone.0143822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y et al (2018) Sunitinib shrinks NET-G3 pancreatic neuroendocrine neoplasms. J Cancer Res Clin Oncol 144:1155–1163. 10.1007/s00432-018-2636-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moertel CG, Hanley JA, Johnson LA (1980) Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N Engl J Med 303:1189–1194. 10.1056/NEJM198011203032101 [DOI] [PubMed] [Google Scholar]
- Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D (1992) Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med 326:519–523. 10.1056/NEJM199202203260804 [DOI] [PubMed] [Google Scholar]
- Muro K et al (2010) Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second-line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 non-inferiority study (FIRIS study). Lancet Oncol 11:853–860. 10.1016/S1470-2045(10)70181-9 [DOI] [PubMed] [Google Scholar]
- Panzuto F et al (2011) Metastatic and locally advanced pancreatic endocrine carcinomas: analysis of factors associated with disease progression. J Clin Oncol 29:2372–2377. 10.1200/JCO.2010.33.0688 [DOI] [PubMed] [Google Scholar]
- Pavel M et al (2016) ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology 103:172–185. 10.1159/000443167 [DOI] [PubMed] [Google Scholar]
- Raymond E et al (2011) Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 364:501–513. 10.1056/NEJMoa1003825 [DOI] [PubMed] [Google Scholar]
- Shibuya H et al (2018) Multi-center clinical evaluation of streptozocin-based chemotherapy for advanced pancreatic neuroendocrine tumors in Japan: focus on weekly regimens and monotherapy. Cancer Chemother Pharmacol 82:661–668. 10.1007/s00280-018-3656-y [DOI] [PubMed] [Google Scholar]
- Shirasaka T et al (1996) Antitumor activity of 1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res 56:2602–2606 [PubMed]
- Turner NC et al (2010) Chemotherapy with 5-fluorouracil, cisplatin and streptozocin for neuroendocrine tumours. Br J Cancer 102:1106–1112. 10.1038/sj.bjc.6605618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y et al (2013) Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab versus S-1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol 14:1278–1286. 10.1016/S1470-2045(13)70490-X [DOI] [PubMed] [Google Scholar]
- Yao JC et al (2011) Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 364:514–523. 10.1056/NEJMoa1009290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JC et al (2008) One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 26:3063–3072. 10.1200/JCO.2007.15.4377 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.