Fig. 5.
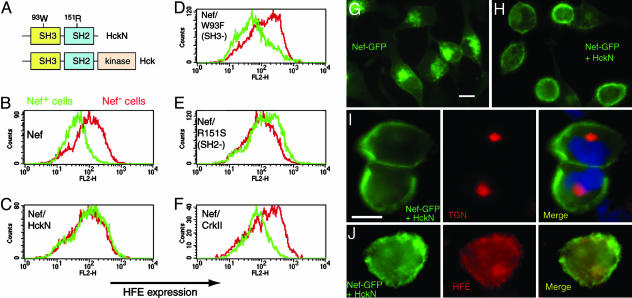
The SH3 domain of dominant negative Hck blocks Nef-mediated down-regulation of HFE. (A) Wild-type Hck has SH3, SH2, and a catalytic domain. Dominant negative HckN lacks the catalytic domain. (B) HeLa-HFE cells transfected with Nef-GFP (green) have reduced surface HFE, compared with Nef-negative cells (red). (C) Cotransfection at 1:1 of Nef-GFP with HckN blocks the effect of Nef on HFE. (D and E) A W93F HckN variant with dysfunctional SH3 domains (D) does not block Nef activity, whereas the blocking effect of HckN is maintained when SH2-disrupted R151S Hck (E) is used. (F) CrkII (which has SH3 domains but does not bind Nef) does not affect HFE down-regulation by Nef. (G) Nef-GFP is normally located perinuclearly, overlapping the TGN, but coexpression of HckN (H) reroutes Nef-GFP to the cell periphery. In the presence of HckN, Nef-GFP no longer colocalizes with the TGN (I, nuclei stained blue with DAPI), and HFE resumes its endosomal localization (J), similar to that in untransfected cells. (Scale bars, 10 μm.)