Abstract
The present study was aimed to cluster sub-groups of patients with varying degrees of cognitive impairment (Subjective Cognitive Decline, mild or Major Neurocognitive Disorder) based on their modifiable risk factors and cognitive reserve with k-means analysis. As a secondary analysis, we described the identified clusters from different perspectives, i.e., socio-demographic characteristics, cognitive functioning, and mental health. The analysis revealed two clusters, which were composed by 27 and 43 patients characterized by protective (Cluster 1) and unprotective (Cluster 2) everyday life habits, respectively. The two groups showed significant differences across all examined dimensions, with Cluster 1 demonstrating a more favourable profile compared to Cluster 2. Specifically, Cluster 1 exhibited advantages in: (1) sociodemographic (education, technological skills, and occupation), (2) cognitive (global cognitive functioning, executive functioning, and working memory), and (3) mental health (mood state and quality of life) characteristics. Such a finding is representative of a more positive individual wellbeing for people who adopt protective behaviours. In the field of dementia prevention, these results support the importance to intervene proactively and simultaneously in the management of multiple risk factors during the entire lifespan.
Subject terms: Psychology, Risk factors
Introduction
The general population’s aging and the increasing number of dementia cases have attracted growing attention to research on prevention of cognitive impairment. In this field, we have assisted to the proliferation of studies aimed to assess the role of the modifiable risk factors (MRFs)1,2—according to the lifestyle-health pathway—in determining the overall wellbeing of elderly population. For instance, Livingston and colleagues1,2 described a life-course model for potential MRFs for dementia, according to which each risk factor could have a different impact on the pattern of age-related changes1,2. In particular, cognitive and social activity, physical functioning, and a Mediterranean diet were found to be positively associated with cognitive health and to account for 20% of the variance in cognitive test scores3. A systematic literature review4 reported that the engagement in cognitive activities over life span has a potential protective role against the development of mild Neurocognitive Disorder (mNCD). In addition, literature highlighted that the adoption of more healthy behaviours—such as following a healthy diet, being physically active, and maintaining social connections—contributes to better mood, reduced anxiety, and better quality of life with benefits to mental health also in late life5–7.
Moreover, a strong association has been established between cognitive reserve (CR) and the trajectories of the effects of the aging process on cognitive functioning, which would result in different resilience levels against neuropathology8. In particular, the relation between age- or disease-related brain changes and cognition is mediated by the construct of CR9, which reflects the ability of using flexibly cognitive processes to compensate for the physiological or pathological deterioration observed in normal and pathological aging, respectively4. The level of CR is mainly determined by the engagement in mental activities, such as undertaking education, working in occupations that require more complex demands, and leisure activities3,10. Hence, CR intervenes moderating the association between pathology and neurological symptoms and buffering the effects of unhealthy MRFs on the clinical manifestations of dementia11,12. For instance, Jia and colleagues12 demonstrated the existence of an interaction between CR and MRFs on dementia, showing that people with low CR have a higher risk of cognitive impairment in case of unhealthy lifestyle profile when compared to a healthy one. However, such an association is still under investigated. To this end, a cluster approach may be useful to evaluate the synergetic impact of healthy factors in older adults with varying degrees of cognitive impairment.
The present study had a main goal, that is, to identify sub-groups of patients based on their MRFs and CR, using a clustering technique. For clustering, we used: (1) the Lifestyle for Brain Health (LIBRA)13,14, which is a risk assessment tool focused on MRFs for dementia in routine care being amenable to represent individual ‘room for improvement’, and (2) the Cognitive Reserve Index questionnaire (CRIq) as a measure of CR15, by combining three proxy indicators: education, working activity, and leisure time. We have intentionally considered CR as a separate factor from the MRFs, given its additive effect on the risk of dementia12. As a secondary analysis, we described how the identified clusters differ from different perspectives, including sociodemographic characteristics, cognitive functioning, and mental health. We hypothesised that the presence of protective MRFs and higher levels of CR would be associated to more positive individual characteristics. By contrast, we expected that patients with unprotective MRFs and lower CR would present a higher prevalence of negative individual characteristics.
Methods
Participants
A total sample of 70 subjects took part in this study. The inclusion criteria for participants were: (a) age > 50 years, (b) education > 5 years, (c) a diagnosis of SCD16, mNCD, or Major Neurocognitive Disorder (MNCD)17 due to Alzheimer’s disease or Vascular dementia, (d) Clinical Dementia Rating (CDR)18,19 score ranging between 0 and 1, and (e) Mini-Mental State Examination (MMSE)20 raw score ≥ 20 (we applied this cut-off to minimize the risk of data unreliability associated with pronounced cognitive impairment).
The exclusion criteria were: (a) presence of cognitive impairment secondary to an acute or general medical disorder (e.g., brain trauma or tumour), (b) presence of severe neuropsychiatric conditions (e.g., mood and behavioural disorders), and (c) presence of severe sensory disorder (e.g., deafness or blindness) or motor functioning deficits in dominant upper limb.
Information about participants profiling in the considered domains were collected through self-report questionnaires and specific standardized instruments. The dataset used for this study was shared on Zenodo platform in accordance with the guidelines of GDPR.
Study design, procedures, and measures
Participants were recruited and enrolled (May 2021–December 2023) from the Dementia Research Center outpatient services and the Neurorehabilitation Unit of IRCCS Mondino Foundation (Pavia, Italy) and screened for eligibility criteria through a clinician evaluation by an experienced neurologist. Once deemed eligible, each participant was contacted to explore their availability and subsequently invited to the Mondino Foundation. The assessment session was conducted in the presence of a neuropsychologist at the Cognitive Psychology Research Section Lab. Each session lasted approximately 120 min, during which anamnesis data were collected, followed by the administration of neuropsychological tests. Self-report questionnaires were then administered at the end of the session. Materials are reported below.
Life style characteristics
Lifestyle for Brain Health (LIBRA)13,14: it is a questionnaire that can help identify and monitor lifestyle risk/protection of dementia by targeting MRFs. The score ranges from -5.9 to + 12.7. Higher scores correlate with higher risk of dementia and cognitive impairment. It investigates the presence or absence of each of the following MRFs evaluated thorough a semi-structured interview: (1) coronary heart disease, (2) diabetes, (3) hypercholesterolemia, (4) hypertension, (5) depression, (6) obesity, (7) smoking, (8) alcohol intake, (9) physical activity, (10) cognitive activity, (11) Mediterranean diet, and (12) renal dysfunction. According to the presence or absence of each MRFs, a specific score has been assigned that concurred to the determination of the LIBRA index, as explained in the Italian validation of this instrument13. In addition to consider the global index, we also treated each MRFs as present or absent, dichotomously.
Cognitive Reserve Index questionnaire (CRIq)15: it estimates CR by means of a collection of participant-related factors. It returns a total score and three sub dimension-related scores, as reported below. Higher scores are indicative of higher CR. The CRIq provides three subscores: (1) CRI-Education refers to the degree of schooling attained by an individual during the life span, (2) CRI-Working Activity records the type and number of years of paid employment held by an individual (different levels of work employment have been identified that differ in the cognitive commitment required as well as the level of responsibility assumed), (3) CRI-Leisure Time refers to all those activities that are usually performed outside the hours of work or school attendance.
Socio-demographic characteristics
An anamnestic interview was carried out in order to collect socio-demographic information, such as age, education, marital and parenting status, past and current occupation, and technological skills.
Cognitive characteristics
We used the following standardized tests to assess five cognitive domains:
global cognitive functioning: MMSE20 and Montreal Cognitive Assessment (MoCA)21;
episodic long-term memory: Logical Memory Test22,23, Rey’s 15 words test immediate-delayed recall24, Rey Complex Figure delayed recall24;
logical-executive functions: Raven’s Matrices 194724, Frontal Assessment Battery25; semantic23 and phonological fluencies (FAS)24, Rey Complex Figure copy26;
working memory: Verbal Span, Corsi’s block-tapping test span22;
attention / processing speed: Attentive Matrices22, Trail Making Test27.
Diagnosis (i.e., SCD, mNCD, and MNCD) was considered as part of the cognitive profile as well.
Mental health characteristics
Beck Depression Inventory (BDI)28: for depressive symptoms. It consists of 21 items that investigate the severity of depressive symptoms. For each set of statements, the subject is asked to choose the one that best describes his/her current situation. The total score is calculated as the sum of the scores of the individual items.
36-Item Short Form Health Survey (SF-36)29: assesses health-related quality of life. It is composed of 36 Likert scale items that return a score related to nine sub-scales: (1) physical functioning, (2) role limitations due to physical health, (3) role limitations due to emotional problems, (4) energy/vitality, (5) mental health, (6) social functioning, (7) bodily pain, (8) general health perceptions, and (9) health changes. Higher scores are indicative of better perceived health status.
Statistical analysis
As primary-outcome measure, we considered patients’ clustering. As secondary-outcome measures, we evaluated clusters in terms of (1) socio-demographic, (2) cognitive, and (3) mental health characteristics.
Clustering is a powerful unsupervised machine learning technique often used in the medical field for discovering hidden patterns across patients’ characteristics that can be difficult to find for medical experts due to the number of variables to be considered simultaneously. We used the k-means clustering algorithm30 that considers n observations and divides them into k different sets such that the sum of distances between the observations and their respective cluster centroid is minimized. In our case, patients were subdivided by the k-means clustering algorithm in two groups according to their CRIq and LIBRA scores. The choice of the number of clusters k can be influenced by prior knowledge of the data or driven by quantitative clustering quality measures such as the mean silhouette score. The mean silhouette score is a measure that considers both cohesion and separation of clusters and ranges between -1 and 1. A value of 1 indicates that the clusters are cohesive and perfectly separated, a negative value indicates that the samples may be in the wrong cluster and a value close to 0 indicates overlapping clusters.
Since mostly of the continuous variables are not normally distributed, non-parametric methods were applied. For the descriptive analysis of the clusters reported in Tables 1, 2, 3, and 4, median and quartiles were provided for all the continuous variables, while the number of participants in each category (n) and the percentage of patients over the total were considered for categorical variables. We used Mann Whitney U test for continuous variables and Chi-Square Test of Independence for categorical variables (Yates’s continuity correction applied). The results of the comparison of the two clusters in the text are reported as W statistic and p value for continuous variables and as 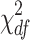 statistic and p value for categorical variables, where are the degree of freedom of the test. The significance level is set as 0.05. Statistical analysis were performed using R 4.4.1.
statistic and p value for categorical variables, where are the degree of freedom of the test. The significance level is set as 0.05. Statistical analysis were performed using R 4.4.1.
Table 1.
Life style characteristics of clusters.
| Cluster 1 (N = 27) |
Cluster 2 (N = 43) |
p-value | |
|---|---|---|---|
| LIBRA MRFs | |||
| Diabetes | 3 (11%) | 8 (19%) | 0.616 |
| Hypercholesterolemia | 11 (41%) | 16 (37%) | 0.966 |
| Hypertension | 7 (26%) | 20 (47%) | 0.142 |
| Depression | 6 (22%) | 14 (33%) | 0.509 |
| Obesity | 1 (4%) | 4 (9%) | 0.683 |
| Smoking | 2 (7%) | 4 (9%) | > 0.90 |
| Physical activity | 13 (48%)* | 6 (14%)* | 0.0043 |
| Cognitively active | 19 (70%)* | 3 (7%)* | < 0.001 |
| Mediterranean diet | 17 (63%)* | 8 (19%)* | < 0.001 |
| Renal disfunction | 0 (0%) | 3 (7%) | 0.426 |
| CRIq | |||
| Education score Median [Q1; Q3] | 109.0 [105.0; 127.0]* | 97.0 [94.0; 109.5]* | 0.002 |
| Working activity score Median [Q1; Q3] | 117.0 [105.5; 130.5]* | 101.0 [93.0; 118.5]* | 0.016 |
| Leisure time score Median [Q1; Q3] | 107.0 [99.0; 112.5]* | 91.0 [81.0; 103.5]* | < 0.001 |
LIBRA = Lifestyle for Brain Health; MRFs = Modifiable Risk Factors; LIBRA = Lifestyle for Brain Health; CRIq = Cognitive Reserve Index questionnaire; Q = Quartile. * denotes significant differences across groups.
Table 2.
Socio-demographic characteristics of clusters.
| Cluster 1 (N = 27) |
Cluster 2 (N = 43) |
p-value | |
|---|---|---|---|
| Age Median [Q1; Q3] | 72.0 [66.5; 76.0] | 75.0 [70.0; 77.0] | 0.222 |
| Sex (female) | 12 (44%) | 25 (58%) | 0.384 |
| Years of education Median [Q1; Q3] | 13.0 [12.0; 18.0]* | 8.0 [8.0; 12.0]* | < 0.001 |
| Technological skills | |||
| None | 3 (11%)* | 15 (35%)* | 0.001 |
| Poor | 0 (0%)* | 9 (21%)* | |
| Modest | 13 (48%)* | 11 (26%)* | |
| Good | 9 (33%)* | 3 (7%)* | |
| Excellent | 2 (7%)* | 2 (5%)* | |
| Missing | 0 (0%)* | 3 (7%)* | |
| Marital status | |||
| Married/cohabitant | 22 (82%) | 33 (77%) | 0.782 |
| Widowed | 4 (15%) | 9 (21%) | |
| Divorced | 1 (4%) | 1 (2%) | |
| Offspring (yes) | 24 (89%) | 41 (95%) | 0.324 |
| Missing | 0 (0%) | 1 (2%) | |
| Current occupation | |||
| Working | 6 (22%) | 5 (12%) | 0.396 |
| Retired | 21 (78%) | 38 (88%) | |
| Past occupation | |||
| Never employed / Low skilled manual worker (e.g., agricultural worker, call center operator, etc.) | 1 (4%)* | 7 (16%)* | 0.007 |
| Skilled manual worker (e.g., craftman, nurse, barber, etc.) | 2 (7%)* | 14 (33%)* | |
| Skilled non manual worker (e.g., white-collar worker, shop keeper, sales representative, etc.) | 12 (44%)* | 16 (37%)* | |
| Professional occupation (e.g., CEO of a small company, psychologist, engineer, teacher, etc.) | 10 (37%)* | 6 (14%)* | |
| Highly responsible or intellectual occupation (e.g., CEO of large company, politicians, university professor, etc.) | 2 (7%)* | 0 (0%)* | |
Q = Quartile. * denotes significant differences across groups.
Table 3.
Cognitive characteristics of clusters.
| Cluster 1 (N = 27) |
Cluster 2 (N = 43) |
p-value | |
|---|---|---|---|
| Diagnostic category | |||
| Subjective Cognitive Decline | 8 (30%) | 4 (9%) | 0.074 |
| mild Neurocognitive Disorder | 18 (67%) | 35 (81%) | |
| Major Neurocognitive Disorder | 1 (4%) | 4 (9%) | |
| Global cognitive functioning Median [Q1; Q3] | 2.7 [1.9; 3.1]* | 2.2 [1.7; 2.4]* | 0.0202 |
| Attention/processing speed Median [Q1; Q3] | 3.0 [2.0; 3.7] | 2.3 [1.3; 3.2] | 0.144 |
| Episodic long-term memory Median [Q1; Q3] | 1.5 [0.9; 2.9] | 1.8 [0.8; 2.5] | 0.725 |
| Logical executive functioning Median [Q1; Q3] | 3.2 [2.3; 3.4]* | 2.6 [1.8; 3.0]* | 0.016 |
| Working memory Median [Q1; Q3] | 3.0 [1.5; 3.5]* | 2.5 [1.5; 3.0]* | 0.038 |
Q = Quartile. * denotes significant differences across groups.
Table 4.
Mental health characteristics of clusters.
| Cluster 1 (N = 27) |
Cluster 2 (N = 43) |
p-value | |
|---|---|---|---|
| Beck Depression Inventory Median [Q1; Q3] | 6.0 [4.0; 14.0]* | 11.0 [8.0; 16.5]* | 0.015 |
| 36-Items Short Form Health Survey | |||
| Physical functioning Median [Q1; Q3] | 85.0 [75.0; 95.0]* | 75.0 [50.0; 90.0]* | 0.019 |
| Role limitations (physical) Median [Q1; Q3] | 75.0 [25.0; 100.0] | 75.0 [12.5; 100.0] | 0.675 |
| Role limitations (emotional) Median [Q1; Q3] | 66.6 [33.3; 100.0] | 66.6 [33.3; 100.0] | 0.609 |
| Energy/vitality Median [Q1; Q3] | 55.0 [50.0; 70.0]* | 50.0 [45.0; 55.0]* | 0.003 |
| Mental health Median [Q1; Q3] | 64.0 [53.0; 74.0] | 64.0 [56.0; 72.0] | 0.607 |
| Social functioning Median [Q1; Q3] | 75.0 [62.5; 93.8] | 75.0 [50.0; 75.0] | 0.118 |
| Bodily pain Median [Q1; Q3] | 75.0 [45.0; 100.0] | 65.0 [45.0; 90.0] | 0.383 |
| General health perceptions Median [Q1; Q3] | 50.0 [45.0; 60.0] | 50.0 [40.0; 65.0] | 0.716 |
| Health changes Median [Q1; Q3] | 50.0 [37.5; 50.0] | 50.0 [25.0; 50.0] | 0.361 |
Q = Quartile. * denotes significant differences across groups.
Results
Cluster analysis
The subjects considered were clusterized with respect to LIBRA and CRIq scores. Since the silhouette scores were really similar between k = 2 and k = 3 (0.405 and 0.412 respectively), the number of clusters was chosen as 2 because of the better interpretability of the results. Twenty-seven and 43 patients composed the resulting clusters. Centroids for Cluster 1 were CRIq = 119.2 ± 16.7, LIBRA = -1.5 ± 1.8; whereas for Cluster 2 were CRIq = 99.9 ± 14.6, LIBRA = 3.0 ± 2.0. The values of the centroids showed that Cluster 1 had higher involvement in cognitive engagement and protective behaviours in everyday life than Cluster 2. When looking at specific MRFs, we found that Cluster 1 included a significantly higher prevalence of individuals physically ( = 8.15, p = 0.004) and cognitively (
= 8.15, p = 0.004) and cognitively ( = 28.06, p < 0.001) active, and following the Mediterranean diet (
= 28.06, p < 0.001) active, and following the Mediterranean diet ( = 12.35, p < 0.001) than Cluster 2. No further differences between Clusters were found in the other MRFs.
= 12.35, p < 0.001) than Cluster 2. No further differences between Clusters were found in the other MRFs.
Cluster 1 had significantly higher CRIq Education (W = 843.0, p = 0.002), CRIq Working activity (W = 781.5, p = 0.016), and CRIq Leisure time (W = 898.0, p < 0.001) scores than Cluster 2. Descriptive statistics as a function of cluster group are reported in Table 1.
Socio-demographic characteristics
Subjects in Cluster 1 were significantly more educated (W = 953.5, p < 0.001), technologically more skilled ( = 18.34, p = 0.001), and with a higher prevalence of participants involved in professional occupations instead of in skilled manual works in the past (χ42 = 14.15, p = 0.007) than Cluster 2. The two clusters were instead similar in terms of age, sex, marital and parental status, as well as current occupation. Descriptive statistics as a function of cluster group are reported in Table 2.
= 18.34, p = 0.001), and with a higher prevalence of participants involved in professional occupations instead of in skilled manual works in the past (χ42 = 14.15, p = 0.007) than Cluster 2. The two clusters were instead similar in terms of age, sex, marital and parental status, as well as current occupation. Descriptive statistics as a function of cluster group are reported in Table 2.
Cognitive characteristics
Clusters showed a tendency to a significant difference in terms of prevalence of diagnostic category ( = 5.20, p = 0.074), with Cluster 1 tending to have a higher prevalence of participants with subjective cognitive decline (SCD) with respect to Cluster 2. Moreover, they significantly differed for global cognitive functioning (W = 387.5, p = 0.020), logical-executive functions (W = 380.5, p = 0.016) and working memory (W = 410.5, p = 0.038), with higher performances for Cluster 1. They did not differ in the other cognitive domains assessed (i.e., episodic long-term memory and attention/ processing speed). Descriptive statistics as a function of cluster group are reported in Table 3.
= 5.20, p = 0.074), with Cluster 1 tending to have a higher prevalence of participants with subjective cognitive decline (SCD) with respect to Cluster 2. Moreover, they significantly differed for global cognitive functioning (W = 387.5, p = 0.020), logical-executive functions (W = 380.5, p = 0.016) and working memory (W = 410.5, p = 0.038), with higher performances for Cluster 1. They did not differ in the other cognitive domains assessed (i.e., episodic long-term memory and attention/ processing speed). Descriptive statistics as a function of cluster group are reported in Table 3.
Mental health characteristics
Cluster 1 had a significantly lower depressive symptomatology (W = 378, p = 0.015), perception of physical functioning (W = 774.5, p = 0.019) and of vitality (W = 821.5, p = 0.003) than Cluster 2. Clusters did not differ in any of the other SF-36 domains. Descriptive statistics as a function of cluster group are reported in Table 4.
Discussion
To the best of our knowledge, this is the first study using a clustering technique to stratify individuals with varying degrees of cognitive impairment based on their everyday life habits. Clusters were defined by two indices: MRFs (assessed with the LIBRA index) and CR (assessed with the CRIq Global score). The main finding of this study is represented by the identification of two clusters with a small within-cluster variation and a maximum between-cluster variation: Cluster 1 associated to protective everyday life habits, and Cluster 2 associated to unprotective everyday life habits.
The exploration of the MRFs mostly associated to the two protective/unprotective clusters showed that Cluster 1 was characterized by a higher prevalence of individuals physically and cognitively active, and who were following a Mediterranean diet. Among all MRFs considered in the LIBRA, these three factors are those that mostly refer to the adoption of preventive behaviours carried out during everyday life and result to be more representative of our clustering. This is in line with broader evidence suggesting the contribute of these factors in delaying cognitive decline31,32: physical activity for vascular and neural health (e.g., Brain-Derived Neurotrophic Factor upregulation)33, cognitive stimulation for cognitive reserve34, and Mediterranean diet in reducing inflammation and oxidative stress35. These findings are further supported by the significant differences we found in all the CRIq indices between the two clusters. This distinct pattern of findings underscores the importance of exploring the synergistic role of MRFs and CR in older adults with varying degrees of cognitive impairment. Conversely, the limited relevance of other MRFs in this clustering may point to the variability in their direct impact or interaction with other factors within this specific sample.
We then examined how the two clusters differed from various perspectives: sociodemographic characteristics, cognitive functioning, and mental health. Cluster 1 was notably more educated, technologically skilled, and had a higher proportion of participants who previously held conceptual occupations compared to Cluster 2. This finding aligns with existing research indicating that higher sociodemographic and socioeconomic status is linked to a healthier lifestyle. This connection likely results from a combination of greater financial resources and a better understanding of the consequences of unhealthy behaviors36–39. It is worth noting that education is considered one of the MRFs within the lifestyle-health pathway, as it represents an early-life factor that influences dementia risk later in life. However, in the context of the present study, we categorized education under participants’ sociodemographic characteristics, as it is unlikely to act as a modifiable factor given the age of our participants.
Regarding cognitive functioning, clusters tended to differ in terms of prevalence of diagnostic categories. Although the p-value did not reach statistical significance and the presence of cases with mNCD or MNCD in Cluster 1 cannot be excluded, some interesting insights can still be drawn. This finding may suggest a potential association between protective and unprotective everyday life habits and the individual characteristics involved in determining the aging trajectory40. Therefore, our results—if confirmed on a larger sample of participants—align with research40,41 indicating that a protective lifestyle assists in better management of neuropathology and in delaying the onset of clinical symptoms of neurodegenerative diseases. More interestingly, clusters differed significantly in terms of global cognitive functioning, logical-executive functions, and working memory, with higher performances for Cluster 1. In line with our results, both healthy lifestyle behaviours and CR were found to mitigate the negative effects of aging on cognitive function42–44. This result is particularly interesting if we consider that logical-executive functions and working memory appear to be particularly involved in quantifying the progression and the risk of dementia45,46 and are target of therapeutic interventions aimed to slow the progression of the disease47. Furthermore, these outcomes could have important clinical relevance, given that logical-executive functions and working memory are known to play a profound role in functional independence in geriatric populations48.
As mental health, we found that Cluster 1 had a significantly lower depressive symptomatology and a better perception of physical functioning and of vitality than Cluster 2. In this field, the existing literature suggests that protective lifestyle habits, such as being active, and adhering to Italian dietary guidelines and recommendations49, are significantly associated with better mental health50,51 and lower depression symptoms in the elderly population52. In addition, CR was found to operate as protective mechanism not only at neural but also at behavioural level by increasing the resilience and adaptability of the brain to cope with geriatric depression and its attendant deficits53. These results are relevant if we consider the association existing between later life depression and dementia due to the involvement of mechanisms such as neuroendocrine changes and hippocampal atrophy54.
This study had some limitations to be considered. Using a clustering technique, we identified sub-groups of patients in the early phases of cognitive decline that were able to characterize themselves in terms of individual wellbeing. However, we used cross-sectional data, hence we could not establish causal relationships between the variables. In addition, the small sample size did not allow us to use a broader number of variables to cluster patients. In this context, we selected those variables that we considered being more representative of the protective/unprotective habits and practices carried out during everyday life. Moreover, our sample was recruited from an outpatient neurological clinic. Hence, this could limit the generalizability of our results to the general population. In particular, it could be expected that subjects attending a clinic may inherently exhibit a lifestyle that is more health-conscious compared to individuals from the general population55. In addition, the fact itself that the two clusters included participants with heterogeneous cognitive status prevented us from fully ruling out the possibility of reverse causality between cognitive status and health behaviors. For all these reasons, future studies are needed to extend and expand our findings, as our results remain interesting and serve as a source of inspiration for further research on larger and multicentric populations.
The present study suggests that it is possible classify individuals with varying degrees of cognitive impairment in two clusters according to the presence of risk and protective factors, which are associated to different levels of individual wellbeing. Hence, we believe that this study has important implications. From a theoretical point of view, stratifying participants based on clusters that account for MRFs and CR can be useful for examining (or controlling for) differences in health and lifestyle factors in epidemiological studies, thus aiding in understanding interindividual differences. From a practical point of view, our results help in reinforcing the concept that dementia prevention should involve the proactive and simultaneous management of multiple risk factors across the entire lifespan course. This is in line with the theory of healthy aging emphasizing how cognitive aging is viewed not as inevitable decline but as a modifiable process influenced by individual and environmental factors, including physical, mental, and social involvement56. Therefore, awareness campaigns that increase understanding of the factors influencing aging trajectories and emphasize the importance of maintaining an active lifestyle even in old age and in the presence of initial decline become critically important.
Acknowledgements
This work was supported by a grant from the Italian Ministry of Health (Ricerca Corrente 2022-2024).
Author contributions
SBe and SBo: Study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript. AV: interpretation of data, drafting of the manuscript. EB and FF: Data Analysis. MP: Interpretation of data. MCR and AC: Patients’ recruitment and data interpretation. EC, TV and CT: Critical revision of the manuscript for important intellectual content.
Funding
This work was supported by a grant from the Italian Ministry of Health (Ricerca Corrente 2022–2024).
Data availability
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: [Zenodo 10.5281/zenodo.11549667].
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was performed in accordance with the guidelines of the Declaration of Helsinki. The study was approved by local ethics committee of San Matteo Hospital, Pavia, Italy (protocol number: P-20210032883, date of approval: May 2021) and all participants signed an informed consent.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet396, 413–446 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livingston, G. et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet404, 572–628 (2024). [DOI] [PubMed] [Google Scholar]
- 3.Clare, L. et al. Potentially modifiable lifestyle factors, cognitive reserve, and cognitive function in later life: A cross-sectional study. PLOS Med.14, e1002259 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbo, I., Marselli, G., Di Ciero, V. & Casagrande, M. The protective role of cognitive reserve in mild cognitive impairment: A systematic review. J. Clin. Med.12, 1759 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hautekiet, P. et al. A healthy lifestyle is positively associated with mental health and well-being and core markers in ageing. BMC Med.20, 328 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassidy, K. et al. Association between lifestyle factors and mental health measures among community-dwelling older women. Aust. New Zeal. J. Psychiatry38, 940–947 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Newman, M. G. & Zainal, N. H. The value of maintaining social connections for mental health in older people. Lancet Public Heal.5, e12–e13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang, H.-X., MacDonald, S. W. S., Dekhtyar, S. & Fratiglioni, L. Association of lifelong exposure to cognitive reserve-enhancing factors with dementia risk: A community-based cohort study. PLOS Med.14, e1002251 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang, H. Nexus between cognitive reserve and modifiable risk factors of dementia. Int. Psychogeriatr.32, 559–562. 10.1017/S1041610220000320 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Bernini, S. et al. Outcomes of a computer-based cognitive training (CoRe) in early phases of cognitive decline: A data-driven cluster analysis. Sci. Rep.13, 2175 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fratiglioni, L., Marseglia, A. & Dekhtyar, S. Ageing without dementia: can stimulating psychosocial and lifestyle experiences make a difference?. Lancet Neurol.19, 533–543 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Jia, F. et al. Cognitive reserve, modifiable-risk-factor profile and incidence of dementia: Results from a longitudinal study of CFAS Wales. Aging Ment. Health25, 2286–2292 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Schiepers, O. J. G. et al. Lifestyle for Brain Health (LIBRA): A new model for dementia prevention. Int. J. Geriatr. Psychiatry33, 167–175 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Franchini, F. et al. The LIBRA index in relation to cognitive function, functional independence, and psycho-behavioral symptoms in a sample of non-institutionalized seniors at risk of dementia. J. Alzheimer’s Dis.72, 717–731 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Nucci, M., Mapelli, D. & Mondini, S. Cognitive reserve index questionnaire (CRIq): A new instrument for measuring cognitive reserve. Aging Clin. Exp. Res.24, 218–226 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Jessen, F. et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dement.10, 844–852 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. (2013).
- 18.Hughes, C. P., Berg, L., Danziger, W., Coben, L. A. & Martin, R. L. A new clinical scale for the staging of dementia. Br. J. Psychiatry140, 566–572 (1982). [DOI] [PubMed] [Google Scholar]
- 19.Morris, J. C. The clinical dementia rating (CDR). Neurology43, 2412 (1993). [DOI] [PubMed] [Google Scholar]
- 20.Measso, G. et al. The mini-mental state examination: Normative study of an Italian random sample. Dev. Neuropsychol.9, 77–85 (1993). [Google Scholar]
- 21.Conti, S., Bonazzi, S., Laiacona, M., Masina, M. & Coralli, M. V. Montreal Cognitive Assessment (MoCA)-Italian version: Regression based norms and equivalent scores. Neurol. Sci.36, 209–214 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Spinnler, H. Italian standardization and classification of neuropsychological tests. The Italian group on the neuropsychological study of aging. Ital J Neurol Sci Suppl8, 1–120 (1987). [PubMed] [Google Scholar]
- 23.Novelli, G., Papagno, C., Capitani, E. & Laiacona, M. Tre test clinici di memoria verbale a lungo termine. Taratura su soggetti normali. Arch Psicol. Neurol. Psichiatr.47, 278–296 (1986). [Google Scholar]
- 24.Carlesimo, G. A. et al. The mental deterioration battery: Normative data, diagnostic reliability and qualitative analyses of cognitive impairment. Eur. Neurol.36, 378–384 (1996). [DOI] [PubMed] [Google Scholar]
- 25.Appollonio, I. et al. The frontal assessment battery (FAB): Normative values in an Italian population sample. Neurol. Sci.26, 108–116 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Caffarra, P., Vezzadini, G., Dieci, F., Zonato, F. & Venneri, A. Rey-Osterrieth complex figure: Normative values in an Italian population sample. Neurol. Sci.22, 443–447 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Giovagnoli, A. R. et al. Trail making test: Normative values from 287 normal adult controls. Ital. J. Neurol. Sci.17, 305–309 (1996). [DOI] [PubMed] [Google Scholar]
- 28.Beck, A. T., Steer, R. A. & Brown, G. Beck Depression Inventory-IIUSA, NCS Person, Inc., Italian Translation: Ghisi, M., Flebus, G.B., Montano, A., Sanavio, E., Sica, C. (2006). Adattamento Ital. Manuale. Giunti O.S. Organ. Spec. (1996).
- 29.Apolone, G. & Mosconi, P. The Italian SF-36 Health Survey. J. Clin. Epidemiol.51, 1025–1036 (1998). [DOI] [PubMed] [Google Scholar]
- 30.Franklin, J. The elements of statistical learning: Data mining, inference and prediction. Math. Intell.27, 83–85 (2005). [Google Scholar]
- 31.Ngandu, T. et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet385, 2255–2263 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Scarmeas, N., Stern, Y., Tang, M., Mayeux, R. & Luchsinger, J. A. Mediterranean diet and risk for Alzheimer’s disease. Ann. Neurol.59, 912–921 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cotman, C. W., Berchtold, N. C. & Christie, L.-A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci.30, 464–472 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Stern, Y. Cognitive reserve. Neuropsychologia47, 2015–2028 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erickson, K. I. et al. Physical activity predicts gray matter volume in late adulthood. Neurology75, 1415–1422 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernini, S. et al. Investigating the individual and joint effects of socioeconomic status and lifestyle factors on mild cognitive impairment in older Italians living independently in the community: Results from the NutBrain study. J. Nutr. Heal. aging28, 100040 (2024). [DOI] [PubMed] [Google Scholar]
- 37.Organization, W. H. Global Action Plan on Physical Activity 2018–2030: More Active People for a Healthier World. (World Health Organization, 2018).
- 38.Deckers, K. et al. Modifiable risk factors explain socioeconomic inequalities in dementia risk: Evidence from a population-based prospective cohort study. J. Alzheimer’s Dis.71, 549–557 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yaffe, K. et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: Prospective study. BMJ347, f7051–f7051 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livingston, G. et al. Dementia prevention, intervention, and care. Lancet390, 2673–2734 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Mondini, S. et al. Cognitive reserve in dementia: implications for cognitive training. Front. Aging Neurosci.8, (2016). [DOI] [PMC free article] [PubMed]
- 42.Phillips, C. lifestyle modulators of neuroplasticity: how physical activity, mental engagement, and diet promote cognitive health during aging. Neural Plast.2017, 1–22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amanollahi, M., Amanollahi, S., Anjomshoa, A. & Dolatshahi, M. Mitigating the negative impacts of aging on cognitive function; modifiable factors associated with increasing cognitive reserve. Eur. J. Neurosci.53, 3109–3124 (2021). [DOI] [PubMed] [Google Scholar]
- 44.Windsor, T. D., Ghisletta, P. & Gerstorf, D. Social resources as compensatory cognitive reserve? Interactions of social resources with education in predicting late-life cognition. J. Gerontol. Ser. B75, 1451–1461 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Pons, A. et al. Utility of the LIBRA index in relation to cognitive functioning in a clinical health seeking sample. J. Alzheimer’s Dis.62, 373–384 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Kirova, A.-M., Bays, R. B. & Lagalwar, S. working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. Biomed Res. Int.2015, 1–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huckans, M. et al. Efficacy of cognitive rehabilitation therapies for mild cognitive impairment (MCI) in older adults: Working toward a theoretical model and evidence-based interventions. Neuropsychol. Rev.23, 63–80 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Overdorp, E. J., Kessels, R. P. C., Claassen, J. A. & Oosterman, J. M. The combined effect of neuropsychological and neuropathological deficits on instrumental activities of daily living in older adults: A systematic review. Neuropsychol. Rev.26, 92–106 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossi, L. et al. The 2018 Revision of Italian dietary guidelines: Development process, novelties, main recommendations, and policy implications. Front. Nutr.9, (2022). [DOI] [PMC free article] [PubMed]
- 50.Harrington, J. et al. Living longer and feeling better: Healthy lifestyle, self-rated health, obesity and depression in Ireland. Eur. J. Public Health20, 91–95 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Bhandari, P. & Paswan, B. Lifestyle behaviours and mental health outcomes of elderly: Modification of socio-economic and physical health effects. Ageing Int.46, 35–69 (2021). [Google Scholar]
- 52.Zhou, W. et al. The relationship between health-promoting lifestyles and depression in the elderly: Roles of aging perceptions and social support. Qual. Life Res.30, 721–728 (2021). [DOI] [PubMed] [Google Scholar]
- 53.Huang, C.-M. et al. Cognitive reserve-mediated neural modulation of emotional control and regulation in people with late-life depression. Soc. Cogn. Affect. Neurosci.14, 849–860 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Byers, A. L. & Yaffe, K. Depression and risk of developing dementia. Nat. Rev. Neurol.7, 323–331 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loef, M. & Walach, H. The combined effects of healthy lifestyle behaviors on all cause mortality: A systematic review and meta-analysis. Prev. Med. (Baltim)55, 163–170 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Depp, C. A. & Jeste, D. V. Definitions and predictors of successful aging: A comprehensive review of larger quantitative studies. Am. J. Geriatr. Psychiatry14, 6–20 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: [Zenodo 10.5281/zenodo.11549667].


