Abstract
Fungi present in Dental Unit Water (DUW) can pose a health hazard to patients and dental personnel. Yet, the issue of fungal contamination of DUW and their conduits, DUW Lines (DUWLs) has been poorly addressed despite a growing body of data on the subject. In this comprehensive review, we aim to address this gap by examining the diverse fungal contaminants found in DUW, the challenges associated with controlling their growth within waterline biofilms, and the various measures employed for fungal decontamination. The review underscores the intricate fungal ecosystems that exist within DUWLs and emphasizes the importance of implementing targeted antimicrobial strategies to uphold waterline hygiene. However, it is important to note that complete eradication of fungi in DUWLs has proven elusive, even with the application of disinfectants at varying concentrations, types, and frequencies. This highlights the pressing need for continued research efforts to develop new and optimized treatment protocols that are specifically tailored to eradicate fungi from DUWLs. Finally, it was notable that there are currently no specific regulations by any dental authority on permissible levels of fungi, as opposed to bacteria, in DUWLs. Legislation developed based on our findings can contribute to the standardization of practices and the formulation of effective control strategies for fungal contamination in DUWLs. It can also guide dental professionals in implementing regular monitoring, proper maintenance, and targeted disinfection protocols to minimize fungal contamination and ensure optimal water quality for patient safety.
Key words: Dental Unit Water Lines (DUWLs), Biofilms, Antimicrobial, Yeasts, Mold
Introduction
Dental Unit Water Lines (DUWLs) refer to the small-diameter tubing integrated within dental chairs that deliver water from either the municipal or an alternative system to the dental instruments.1 They play a vital role in numerous dental procedures, including instrument cooling, oral rinsing, and the emission of an air/water spray that aids in rinsing away debris, blood, saliva, or dental materials during treatment procedures. By doing so, they contribute to the maintenance of a clean operative field, ensuring effective dental care.2,3
Under normal circumstances, water delivered via DUWL is typically siphoned and removed through efficient vacuum suction units integrated into the chair mechanism. However, it is inevitable that some water may be swallowed unintentionally due to reflective deglutition, particularly due to poorly developed reflexes in young children, and handicapped patients. In addition, airborne water micelles and droplets emanating from DUW may be inhaled by those in the proximity of the operative field, including the patients and dental personnel. Hence, it is crucial that the water quality in DUWLs meet specific standards. Ideally, the water should be of potable quality like those delivered to households via municipal supplies in developed countries such as the USA and Europe.4,5
The standards for water quality in DUWL have been promulgated by organizations such as the American Dental Association and the Department of Health in the UK.6,7 ADA recommends that dental offices follow the Centers for Disease Control and Prevention (CDC) guidelines, which state that DUW used for nonsurgical procedures should have a colony-forming unit (CFU) count of less than or equal to 500 CFU/mL of heterotrophic bacteria. In the United Kingdom, the Health Technical Memorandum 01-05 (HTM 01-05) published by the Department of Health has stated that the acceptable microbiological water quality for DUWLs should be less than or equal to 100 CFU/mL of heterotrophic bacteria. Interestingly both of these standards primarily focus on bacterial contamination and do not specifically mention permissible numbers of fungi.
Fungal contamination in DUWLs is a complex issue, and there is currently no universally accepted standard or guideline for permissible fungal levels in dental water. This may be due to several factors, as can be seen in the current review. These include the wide variety of fungal species that can be present, the challenges in accurately quantifying fungal load, and the varying pathogenicity and health risks associated with different fungal species.
In this manner, adhering to guidelines and standards for potable water quality helps safeguard the health and well-being of both patients and dental professionals. Regular monitoring and appropriate measures to control microbial contamination are hence necessary to uphold these standards and promote a safe dental environment.
Microorganisms within DUWLs can exist in two distinct phases. First, they can be present in the luminal water in a suspended state, in the planktonic form. In this state of existence, the microbes are freely floating in the water and can be easily carried through the system. Second, microbes can be attached to the surfaces of water tubing within biofilms, existing in a sessile form. Biofilms are complex microbial communities that develop on the inner surfaces of DUWLs. Within these biofilms, microorganisms form structured colonies encased in a self-produced matrix, that provides protection and facilitates their attachment to the tubing surfaces.8
Biofilms develop due to a combination of factors. The narrow diameter and the extensive surface area of the tubing combined with the low flow rate, contribute to water stagnation in DUWLs which provides an ideal breeding ground for microbial growth, ultimately leading to biofilm formation.8,9 The resident flora in these biofilms have the ability to detach and contaminate the water, creating a potential risk of infection. While the minimum infectious dose for fungi is generally higher than for bacteria, studies show that even low-level fungal contamination can pose significant health risks, particularly for immunocompromised individuals. Such contamination may occur through inhalation or direct contact with nonintact skin or mucous membranes of the exposed individual.
Pathogens such as Legionella spp., nontuberculous Mycobacteria, and Pseudomonas, yeasts, filamentous fungi spp., protozoans,8 are now known to colonize DUWL biofilms together with harmless saprophytic bacteria.1,8 Hence it is crucial to address biofilm formation and implement effective measures to prevent microbial contamination and minimize the risk of infection transmission in dental settings.
A comprehensive and integrated approach is required to address microbial contamination in DUWLs. This includes engineering controls, chemical controls, and procedural components.9 Engineering controls are crucial as the first line of defence.10,11 In brief, engineering controls include integrated water reservoirs, in-line microbial filters, and antiretraction valves that are installed by manufacturers to minimize the ingress of contaminants and provide a strong foundation for maintaining water quality and preventing microbial contamination.10 Procedural controls are equally important and should be followed diligently. These include regular flushing and drying of the lines, strict adherence to maintenance routines, and consistent water quality monitoring. Finally, chemical controls entail the application of formulated disinfectants and biocides into the water reservoirs of the dental chairs.1,10 By implementing a comprehensive approach that combines engineering, procedural, and chemical controls, dental practices can effectively address microbial contamination in DUWLs.8,12,13
Despite these control systems, biofilm develops within DUWLs, particularly when there are operational lapses. A number of reports now indicate that, of the microbes that inhabit the DUWL biofilms fungi are particularly resistant to these control measures. The intricate hyphal network of fungi creates a protective environment, effectively shielding the entire microbial community within the biofilm while their mycelial structure significantly contributes to the biofilm integrity and resilience.8,14,15 Effective decontamination is also challenging as biofilms can rapidly reform after removal, and continuous exposure to sublethal doses of antimicrobial agents can lead to increased resistance.16 While the minimum infectious dose for fungi is generally higher than for bacteria, studies show that even low-level fungal contamination can pose significant health risks, particularly for immunocompromised individuals.17,18 Inhalation of fungal contaminants of DUWLs, especially by compromised patients, may lead to opportunistic infections with the potential to cause fungemia, endocarditis, and systemic candidiasis.8,14,19
A careful review of the English language literature has indicated that there is a significant gap in reviews appertaining fungal contamination and decontamination of DUW. Therefore, this review aims to address this gap by examining the prevalence, diversity, and load of fungi in DUWL. The opportunity was also taken to systematically evaluate the fungal persistence in DUWLs following exposure to disinfectants administered through different approaches.
Material and methods
Data sources
Two investigators (NS and KSF) performed an electronic search of English language manuscripts using the Medline database via OVID, Embase, and Web of Science. The search encompassed published cross-sectional surveys and intervention studies available in the literature from January 1990 to November 2023.
Primary objective
To systematically review the data on the prevalence, diversity, and load of fungi in DUWLs.
Secondary objective
To systematically review and analyse the available data on the mechanisms contributing to fungal proliferation within DUWLs, and the effectiveness of chemical and nonchemical approaches for decontaminating DUWLs.
Study selection
Inclusion criteria
-
-
Studies reporting fungal presence in planktonic or sessile form in DUW sources or reservoirs in public or private clinics, Dental Hospitals, and Teaching Dental Hospitals.
-
-
Studies on the microbiological assessment of fungi (yeasts/filaments or moulds) before and after clinical operations in the DUWLs.
-
-
Studies with data on the fungi profile of DUWLs across different Dental Units operating within various specialties of Dentistry.
Exclusion criteria
-
-
Studies without fungal contamination data in DUWLs.
-
-
Review articles on fungal contamination in DUWLs.
-
-
Reports that did not permit data extraction necessary to achieve the study objectives.
-
-
Studies with incomplete outcome details relevant to fungal contamination in DUWLs.
-
-
Poster presentations, conference abstracts, and unpublished materials.
-
-
Grey literature and unpublished information.
Overall summary measure
The main outcome was to systematically review the evidence of fungal contamination in DUWLs and the effectiveness of various chemical approaches for fungal decontamination of DUWLs.
Electronic data search, data extraction, and analysis
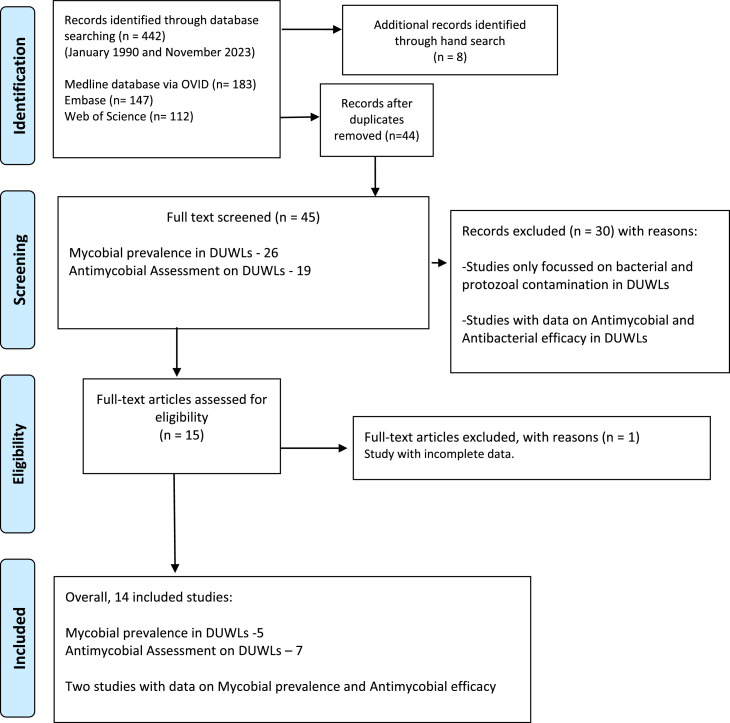
The systematic procedure adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Scoping Reviews criteria20 to ensure a comprehensive and standardized approach. Figure 1 provides a summary of the applied search strategy and outcomes.
Fig.
PRISMA flow chart of the literature search and study selection.
The electronic data search and analysis were conducted in three distinct steps. In the initial stage, two reviewers (NS and KSF) evaluated the titles and abstracts of all identified studies that met the preset inclusion criteria. A third reviewer (LPS) cross-checked the evaluations for consensus. Full-text analysis was carried out when all the necessary information for making informed decisions was available. During the full-text review, the researchers (NS and KSF) meticulously examined the eligibility criteria and documented the results relevant to the study's objectives in spreadsheets for easy retrieval.
Furthermore, a comprehensive search of the references provided in the included publications was conducted to identify additional research for evaluation.
The third stage involved data extraction and assessment. The reviewers meticulously documented detailed information on each study characteristics, including the study design, country, type (biofilm/water samples), site of DU, specimen preparation, and tested fungi (moulds/yeasts). Information regarding the type of intervention, comparator, evaluation time, evaluation techniques, and study results was also analysed. This rigorous process ensured the systematic collection and analysis of relevant data from the identified studies.
The identified research papers meeting the inclusion criteria were categorized using bibliographic software, EndNote version 11 (Clarivate Analytics).
Quality and the overall risk of bias assessment of the included reports
During the fourth stage of the systematic review, two reviewers (NS and KSF) independently assessed the methodological quality of the eligible studies. The methodological quality of the study evaluating the prevalence and enumeration of fungal load in DUWLs was conducted by assessing the risk of bias using the Cochrane Collaboration risk of bias assessment tool. This evaluation covered the study's randomization process, allocation concealment, blinding of outcome assessment, selective reporting, and other potential biases. Disagreements were resolved through discussion until the reviewers (NS, KSF, and LPS) reached a unanimous agreement, categorizing the study's risk of bias as low, unclear, or high, as outlined in Tables 1 and 2.
Table 1.
Risk of bias assessment of the studies evaluating fungal prevalence and enumeration in DUWLs.
| Studies | Representative selection Did the authors describe a method to represent water or biofilm samples accurately? | Randomized sampling Was sampling randomized? |
Quality control Were procedures for ensuring the quality of dental unit waterlines specified or mentioned? |
Methodology Are clear and suitable microbial analysis methods described or mentioned? |
Point of sampling Was the water sampling point specified, such as from an air/water syringe or high-speed handpiece? |
Sampling handling criteria This includes the collection and transportation of water and biofilm samples. |
Description of sample handling | Description of analytical method/s for microbial prevalence and count | Peer review publication | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|
| Błaszczyk et al23 | × | × | × | × | × | × | - | × | × | H |
| Fan et al24 | × | × | - | × | × | - | - | × | × | M |
| Omran et al25 | × | - | - | × | × | - | - | × | × | M |
| Mazari et al26 | × | - | - | × | × | - | - | × | × | M |
| Damasceno et al29 | × | × | - | × | × | - | - | × | × | M |
| Kadaifciler et al27 | × | × | × | × | × | × | - | × | × | H |
| Szymańska28 | × | × | - | × | × | - | - | × | × | M |
x =yes; - =no; Low quality (score less than three); Medium quality (score between three and six); and High quality (score more than six).
Table 2.
Risk of bias assessment of the studies evaluating efficacy of antifungal agents in DUWLs.
| Studies | Selection bias Baseline characteristics similarity/appropriate control selection |
Selection bias Allocation concealment |
Selection bias Randomization |
Performance bias Blinding of Researchers |
Detection bias Blinding of outcome assessors |
Reporting bias Selective outcome reporting |
|---|---|---|---|---|---|---|
| Hussain Akbar et al33 | + | + | + | - | ? | ? |
| Omran et al25 | + | + | - | - | ? | ? |
| Fujita et al34 | + | + | + | + | + | + |
| Costa et al35 | + | + | + | + | ? | ? |
| Szymańska28 | + | + | + | + | ? | ? |
| Porteous et al39 | + | + | - | + | ? | ? |
Risk of bias assessment legends: + (Low risk); - (High risk); ? (Unclear risk).
The final scores were allocated using a checklist derived from a systematic review conducted by Bain et al, in 201421 and adapted by Bayani et al, in 202322 focusing on the prevalence of contamination in water lines. The score range for the checklist extended from zero to nine. The reviewed articles were then categorized into three levels based on their quality: low quality (score less than three), medium quality (score between three and six), and high quality (score more than six), as shown in Tables 1 and 2.
Results
Prevalence of fungi in DUWLs
The reviewed studies, conducted across eight countries in different continents, clearly provide compelling evidence for the global prevalence of fungi in DUWLs.23, 24, 25, 26, 27, 28, 29 These studies encompassed a wide range of dental settings, including hospital dental clinics, private practices, and dental educational institutions, covering a total of 223 dental units. The analysis comprised an extensive dataset of 79 biofilm and 592 water samples, Tables 3 and 4.
Table 3.
Prevalence of yeasts and molds in DUWLs.
| Studies | Yeasts | Molds |
|---|---|---|
| Fan et al24 |
Candida spp., Exophiala (Exophiala is categorized as a mould, yet it's distinct for having a yeast-like form among its group of fungi) |
Aspergillus, Penicillium, Cladosporium, Alternaria, Fusarium, Stachybotrys |
| Omran et al25 | Candida spp. | Aspergillus flavus |
| Mazari et al26 |
Candida albicans C. guilliermondii C. glabrata Rhodotorula spp., Trichosporon spp. |
|
| Kadaifciler et al27 |
Candida famata, C. guilliermondii Cryptococcus laurentii |
Aspergillus pseudoglaucus Cladosporium spp., Penicillium spp., Penicillium verrucosum, P. decumbens, P. waksmanii, Acremonium spp. |
| Szymańska28 |
C. albicans, C. curvata, Geotrichum candidum |
Aspergillus amstelodami, A. fumigatus, Aspergillus spp. from Aspergillus glaucus group, A. repens, Citromyces spp., Penicillium aspergilliforme, P. pusillum, P. turolense, Sclerotium sclerotiorum |
| Błaszczyk et al23 |
Candida parapsilosis Rhodotorula spp., |
Cladosporium spp., Alternaria spp., Penicillium spp., Fusarium oxysporum |
| Damasceno et al29 |
Candida spp. Rhodotorula minuta R. rubra |
Aspergillus spp. Fusarium spp. Penicillium spp. Trichoderma spp., Acremonium spp., Bipolaris spp., Rhinocladiella spp. |
Table 4.
Prevalence of fungal biofilms in the DUWLs.
| Studies, country | Site | No. of DUWLs biofilm samples | Sampling site | Microbiological assessment | Genus/species total number of strains |
|---|---|---|---|---|---|
|
Błaszczyk et al23 Poland |
34 dental units from academic dental polyclinic (9 surgical, 17 conservative, eight periodontal) | NM |
Swabs from dental unit parts (eg, as an air/water syringe, a scaler, a turbine, a micromotor, an inner surface of the bottle under the dental chair, and a spittoon tap) Water samples from the bottle under the dental chair, a cup intended for the patient, a container in the room, a main bucket of the polyclinic, and water used for supplying all dental workstations, excluding surgical ones |
Culture method (Columbia Agar with 5% sheep blood, McConkey medium, Sabouraud Dextrose with chloramphenicol LAB-AGAR Manual enumeration CFU/mL MALDI-TOF MS mass spectrometry for identification |
Mould fungi in 27/34 periodontal units and some conservative units Cladosporium spp. in 6/9 surgical units Surgical units: Total fungi contamination: 20,400 CFU/mL (10 lowest, 19,900 highest) Rhodotorula spp., (highest) Cladosporium spp., Alternaria spp., Penicillium spp., and Fusarium oxysporum Conservative units: total count 140 CFU/mL (40 lowest, 100 highest) Candida parapsilosis Periodontal units: total 350 CFU/mL (lowest 10, highest 340) Alternaria spp. and Penicillium spp. |
|
Fan et al24 China |
Dental Hospital | 36 biofilm samples 18 DUWLs of six specialties: Prosthodontics, Orthodontics, Paediatrics, Endodontics, Oral surgery Periodontics At two time points (ie, before and after routine clinics) |
Plastic tubes connected to high-speed handpiece | NGS methods (16S ribosomal DNA (rDNA) (V3-V4 regions) and ITS2 gene were sequenced) | Dominant genera in the prosthodontics, orthodontics, and endodontic clinics DUWLs were Aspergillus, Candida, and Purpureocillium Paediatric clinics DUWLs were Aspergillus and Purpureocillium Oral surgery and periodontics were Aspergillus and Candida Aspergillus was the dominant genus of mould among all groups. Six potentially pathogenic genera were identified: Aspergillus, Candida, Penicillium, Cladosporium, Alternaria, Fusarium, Exophiala, and Stachybotrys |
|
Omran et al25 Egypt |
82 dental units, (20 dental units from three private dental clinics) 62 dental Units from nine Government Hospitals |
204 water samples from DUWLs outlets At two time points (the beginning and end of the clinical sessions) |
N = 71 water samples from high-speed handpieces N = 66 water samples from air/water syringes, N = 67 water samples from cup filler |
The enumeration of yeast counts as CFU/mL. Fungal identification via API Candida system and MALDI-TOF MS Biofilm colony forming ability |
Aspergillus flavus, Candida spp. (Candida dubliniensis were 9 out of 10 yeast isolates) mean (±SD) CFU/mL High-speed handpiece (n = 71) 39.5 ± 97.0 Air/water syringe (n = 66) 38.8± 97.6 Cup filler (n = 67) 17.2 ± 48.5 At the beginning of the clinical session, all isolates were of Aspergillus flavus (moulds) At the end of the clinical session, moulds decreased significantly, and samples were contaminated by Candida spp. |
|
Mazari et al26 Algérie |
18 DUWLs of the University Dental Hospital | 18 biofilm samples (Dental clinic = 13) (Stomatology unit = 5) |
NM | Culture method (Sabouraud dextrose agar, CHROMagar Yeast identification system Api Candida, MALDI-TOF MS DNA sequencing |
Eleven strains of Candida species C. albicans (2), C. guilliermondii (5) C. glabrata (4) Rhodotorula spp. (1) Trichosporon spp. (2) |
|
Damasceno et al29 Brazil |
5 DUs from the surgical clinic and periodontics clinic at Dental College in duplicate |
240 samples altogether Three series of samples: deionized water, sterile water, and deionized water after exchanging plastic DWULs connections for new ones |
Water samples from the central water reservoir, dental unit water reservoir, the water of triple syringe, the water of high revving engine | Culture on R2A agar to count the colonies and subculture on Sabouraud Dextrose plus chloramphenicol for further identification. Morphological identification, germ tube test, presence of chlamydospores on Tween 80 meal agar (Difco), zymogram, auxanogram, CHROMagar Candida Additionally, susceptibility to fluconazole and the ability of collected fungi to form biofilm were assessed |
The number of colonies ranged from 0 to 40 CFU/mL. Central water reservoir 6.80 ± 11.14 Water reservoirs of the dental units 4.20 ± 7.67 Triple syringes 4.00 ± 7.44 High-revving engine 14.93 ± 18.18 Fungal species observed: Aspergillus spp. Fusarium spp. Penicillium spp. Rhodotorula minuta Rhodotorula rubra Candida lusitaniae C. guilliermondii Trichoderma spp., Acremonium spp., Bipolaris spp., and Rhinocladiella spp. Aspergillus spp., Fusarium spp. (except isolate no. 1, during a 72-h period) and R. rubra and R. minuta were shown to form a biofilm R. rubra and R. minuta strains were 100% resistant to fluconazole, C. guilliermondii and C. lusitaniae strains were approximately 80% sensitive |
|
Kadaifciler et al27 Turkey |
41 DUWLs of 21 private dental and 20 public dental clinics | 123 water samples | 250 mL water samples collected from air-water syringes, high-speed drills, and inlet waters in the morning before patients’ treatment | Culture method The enumeration of yeast counts as colony-forming units per millilitre (CFU/mL). Gram staining, germ-tube formation, and biochemical tests (Carbohydrate assimilation [API 20C AUX system] for yeast identification) |
Isolated fungi were Penicillium waksmanii, Cladosporium spp., Penicillium spp. Candida famata, Cryptococcus laurentii, C. guilliermondii, Penicillium verrucosum, Aspergillus pseudoglaucus, Penicillium decumbens, and Acremonium sp. The aerobic mesophilic microbial count in high-speed drills was higher than in inlet waters and air-water syringes Some fungal genera were established only in the outlet of DUWLs. Nonsporulating fungi were found in 7 dental units |
|
Szymańska28 Poland |
25 dental units of the public dental clinics | 25 water samples 25 biofilm swab samples |
Water samples from the water reservoir of a dental unit water system Water samples from the high-speed handpiece Biofilm sample from the wall of the main waterline provides water to dental handpieces |
Culture method on malt agar | In water from high-speed handpieces, fungi were detected in 16 of 25 samples. In biofilm samples from the DUWL main tubes fungi were observed in 11 of 25 swab samples Overall, the following fungi were found: Aspergillus amstelodami, A. fumigatus, Aspergillus spp. from Aspergillus glaucus group, Aspergillus repens, Citromyces spp., Penicillium aspergilliforme, Penicillium pusillum, Penicillium turolense, Sclerotium sclerotiorum Candida albicans, C. curvata Geotrichum candidum Reservoir water: Candida curvata, C. albicans, and Aspergillus glaucus Swab sample: Aspergillus glaucus group, C. albicans, and Sclerotium sclerotiorum Water from a high-speed handpiece: C. albicans, Aspergillus glaucus group, Sclerotium sclerotiorum |
DU, dental units; DUWLs, dental unit water lines; NM, not mentioned.
This broad dataset revealed a diverse range of fungal species within DUWLs across various dental disciplines, including surgical, periodontal, prosthodontic, and paediatric dentistry units. The prevalence of fungi was widespread, affecting multiple components of the DUWLs system, including handpieces, triple syringes, ultrasonic scalers, tap water, reservoir bottles, and the main water reservoirs. This indicates a pervasive distribution of fungi within these dental environments.
Moreover, the data indicated an extensive array of approaches used by various workers to detect fungal presence in DUWLs. These varied from advanced diagnostic technologies, such as Next-Generation Sequencing and MALDI-TOF Mass Spectrometry, to more traditional fungal isolation and identification systems, such as the API Candida system, to straightforward culture methods and microscopic imaging.
Some studies reported sample collection at two specific time points: at the commencement and the conclusion of clinical sessions,24,25 or singly prior to patient treatment.27 Analyses of DUWLs in surgical units revealed a significant prevalence of fungal presence23,24 and this was followed by specialties in Periodontics, Prosthodontics, and Pedodontics. Furthermore, among the different components of DUWLs, water samples from high-speed handpieces, followed by those from air-water syringes, exhibited a pronounced fungal presence.25,27, 28, 29
In terms of the fungal genera and species identified Candida spp., were the frequent contaminants,23, 24, 25, 26, 27, 28, 29 across multiple reviewed studies. Other notable yeast genera isolated were Rhodotorula spp., and Trichosporon spp.23,26,29
Of the Candida species C. albicans were the commonest isolates followed by C. guilliermondii, C. glabrata, and C. parapsilosis. They are well-known for their association with candidiasis, which can manifest as mucosal infections such as oral thrush, as well as systemic infections, particularly in individuals with compromised immune systems.30 The least common yeast species identified were Geotrichum candidum and Cryptococcus laurentii,27,28 which may pose health risks, particularly the latter, in immunocompromised patients.31 Various moulds including, Aspergillus, Penicillium, Cladosporium, Alternaria, Fusarium, and Stachybotrys spp., have been identified by various investigators in the reviewed studies.23, 24, 25,27, 28, 29 These moulds are of concern due to their ability to produce mycotoxins and trigger allergic reactions and asthma. Aspergillus, which was prevalent in almost all reviewed studies, is noteworthy as it can cause serious infections known as aspergillosis, especially in individuals with weakened immune systems.32 Additionally moulds such as Acremonium, Bipolaris, and Rhinocladiella spp., moulds,27,29 which are less common but can cause opportunistic infections have also been reported. These findings underscore the importance of effectively managing the prevalence of both yeasts and moulds in DUWLs.
Methods employed to control fungi in DUWLs and their effectiveness
The reviewed dataset outlined various antimicrobial agents used for treating DUWLS including the proprietary, Poseidon-S system (electrolyzed chlorine water), Calbenium, Oxygenal 6, Sterispray, chlorine, and hydrogen peroxide.25,28,33, 34, 35, 36, 37, 38, 39 Treatment period varied amongst studies, ranging from 5 minutes34 to overnight39 or continuous28 presence in the DUWLs, depending on the specific agent and the study conditions. The effectiveness of these treatments in reducing fungal presence in DUWLs was assessed using different methodologies. These included total viable count, quantitative polymerase chain reaction, and pyrosequencing of 18S rRNA gene sequences. The outcomes of these studies demonstrated varying degrees of efficacy in reducing the fungal and yeast burden in DUWLs. It is important to note that the specific results and efficacy of each antimicrobial agent varied depending on the study design, treatment protocols, and the characteristics of the DUWL system under investigation implying the need for further research and optimization of treatment strategies to eradicate fungi in DUWLs. The reviewed data encompasses a wide range of settings in multiple countries, including dental hospitals, clinics, and laboratories. The types and numbers of samples varied too, including water samples, biofilm samples, and those targeting only specific fungi such as C. albicans34,37 or all yeasts and moulds,25,28,35,36 in either planktonic37 and sessile states35,36 or a combination of both.38 Characteristics of samples, too, were diverse, focusing on different fungal species collected from various dental unit components. The dataset evaluates the efficacy of various antifungal treatments applied to DUWLs. The outcome of these differed based on the type of treatment type, concentration, and the duration of the exposure to the disinfectants used Table 5. Some workers specifically focused on evaluating the effect of antimycotic agents on Candida spp. only. For instance, Fujita and colleagues34 observed that electrolyzed chlorinated water effectively eliminated C. albicans growth within 5 minutes when treating 20-year-old dental units. On the contrary, Hussain et al,33 observed a reduction in Candida growth on SDA agar following a 10-minute treatment of biofilm samples with Oxygenal 6 (w/v) of H2O2 and silver ions. This protocol included flushing water tubing and triple syringes of newer dental units (A-dec and KaVo Units), suggesting the potential for reducing specific yeast colonization through specific treatment protocols. However, it should be noted that these studies may have limitations regarding the age and maintenance of the tested Dental Units. Furthermore, it is important to acknowledge that these studies focused specifically on Candida and the results may not necessarily apply to other types of fungi or moulds. This possibility was shown by the work of Costa et al36 who reported the limited efficacy of antimycotic applications on 8- to 12-year-old DUWLs. They observed that despite flushing and application of disinfectants (Calbenium/Oxygenal 6), over 80 distinct fungal genera persisted in DUWLs. The residual drug-resistant fungi in SUWLs were predominantly the Ascomycota and Basidiomycota phyla. In particular, the Saccharomycetes belonging to Ascomycota phylum was the dominant species within the core fungal microbiome together with Candida species in all three examined DUWLs. Regarding the duration of disinfectant application, two studies evaluated the antifungal effect of hydrogen peroxide and sodium hypochlorite at varying concentrations and durations. Omran et al25 compared daily and weekly applications of hydrogen peroxide (0.2%) and sodium hypochlorite (at concentrations of 5.0%, 10.0%, 15.0%, and 20.0%) for disinfecting DUWLs. They found no significant difference in the efficacy of the two application frequencies. However, dental units using 0.2% hydrogen peroxide and the highest concentration (20.0%) sodium hypochlorite exhibited demonstrated greatest decontamination efficacy.
Table 5.
Antimycotic effects of various disinfectants in DUWLs.
| Studies, country | Setting | No. of samples sample characteristics | Antimicrobials agent/s treatment time | Measurement of antimycotic effect | Overall outcome |
|---|---|---|---|---|---|
| Clinical Settings | |||||
|
Hussain Akbar et al33 Kuwait |
12 dental units, 6 A-dec, and 6 KaVo, two water and one biofilm sample were collected before and after disinfection from a high-speed handpiece, water source, and 5 cm piece of tubing | Candida species (not specified) |
Oxygenal 6 6% (w/v) of H2O2 and silver ions Used to treat water storage bottles, flushing dental unit water tubing, and syringes 10 min |
Sampling on Sabouraud Dextrose Agar (SDA) Mean (SD) CFU counts after 2 wk of introducing the treatment |
Decrease in Candida colonies on SDA agar biofilm samples after treatment. |
|
Omran et al25 Egypt |
82 DUs (62 from Government hospitals, 20 from private clinics). Samples from high-speed handpieces, air/water syringes, and cup fillers collected before and after work day |
Aspergillus flavus, Candida dubliniensis |
Hydrogen peroxide 0.2% Sodium hypochlorite 5.0%, 10.0%, 15.0%, 20.0% Daily or weekly |
CFU/100 mL Membrane filtration was used, and membrane filters were cultured on Rose Bengal Chloramphenicol (RBCh) |
Daily vs weekly application of disinfectant was not significant. DUs using 0.2% H2O2 and 20.0% sodium hypochlorite had the highest acceptability rates, contrary to those with 5% sodium hypochlorite. Samples showing fungal growth were all from DUs treated with sodium hypochlorite. |
|
Fujita et al34 Japan |
2 dental units at the dental hospital had been in use for 20 y | C. albicans |
Poseidon-S system- an electrolysis apparatus that uses only the chlorine employing the microbicidal effects of the electrolyzed water 5 min |
Total viable count (TVC) in CFU/mL | No growth of C. albicans was observed after treatment with P-water (electrolyzed water). |
|
Costa et al35 France |
Three 8- to 12-y-old dental units | Water samples DU1 and DU2 = public clinic DU3 = hospital clinic Were assessed for fungal diversity in the old dental unit's water lines treated with 2% Calbenium and 0.3% Oxygenal 6 |
Calbenium (Ethylene diamine tetra acetic acid [EDTA], ammonium IV, sodium tosylchloramide, allantoin, aspartame, sorbitol, and aroma) Oxygenal 6 6% (w/v) of H2O2 and silver ions DUWL of dental units 1 and 2 treated with 2% of Calbenium DUWL of dental unit 3 with 0.3% of Oxygenal 6 For Unit 3, an additional treatment each week on Friday consisted of a cycle of 45 min with Oxygenal 6 at 3% (v/v) circulated inside DUWL |
Water sample tested via culture method (CFU/mL) Quantitative polymerase chain reaction (qPCR) Pyrosequencing 18S rRNA gene sequences |
Despite flushing and disinfectant applications, more than 80 distinct fungal genera colonize the DUWL, mainly belonging to Ascomycota (including both yeasts and moulds) and Basidiomycota phyla. The saccharomycetes class (part of the Ascomycota phylum and primarily composed of yeasts) was dominant in the core fungal microbiome of DUWL of 3 dental units. The Candida genera occurred in all three studied DUWL of the tested dental units. |
|
Szymańska28 Poland |
Water samples from dental unit reservoirs, highspeed handpieces, and biofilm from the waterline walls of dental units |
Aspergillus amstelodami, A. fumigatus, Aspergillus spp. from Aspergillus glaucus group, Aspergillus repens, Citromyces spp., Penicillium aspergilliforme, Penicillium pusillum, Penicillium turolense, Sclerotium Sclerotiorum Candida albicans, C. curvata, Geotrichum candidum |
First stage: 0.25% hydrogen Peroxide for 30 min Second stage: Constant presence of 0.02% hydrogen peroxide in DUWL 0.25% hydrogen Peroxide for 30 min |
CFU/mL | Hydrogen peroxide caused a significant decrease in the number of total fungi and individual fungal species. |
|
Porteous et al39 USA |
Six dental unit water lines pooled samples from tap water (main source); handpieces, ultrasonic scalers Vs Water lines in control dental units |
Exophiala mesophila | At the end of the clinic operation, a chlorine dioxide compound (100 mL) was run through the lines and left overnight Overnight |
The water sample was tested via the culture method on R2A agar CFU/mL for each dental unit each week for 15 wk |
Treatment of DUWLs with a continuous-use waterline cleaner may alter the natural water flora and promote the growth of a fungus already present in small amounts in the municipal water supply. |
| Laboratory settings | |||||
|
Costa et al36 France |
Laboratory | C. albicans (in polymicrobial biofilms containing Pseudomonas aeruginosa and Vermamoeba vermiformis) |
Calbenium (Ethylene diamine tetra acetic acid [EDTA], ammonium IV, sodium tosylchloramide, allantoin, aspartame, sorbitol, and aroma) Oxygenal 6 6% (w/v) of H2O2 and silver ions Sterispray (Benzalkonium chloride, EDTA, chloramine, aspartame, sorbitol, aroma, and essential thyme oil) 15 min |
Enumeration of culturable mycotic cells | Calbenium and Sterispray eradicated C. albicans cells after a 15-min treatment using concentrations of at least 0.5% and 3%, respectively. Stagnation contributed to the persistence of fungal cells in biofilms but did not promote their multiplication after using disinfectants. |
|
Petti et al37 Italy |
Laboratory | NM Freshly isolated planktonic C. albicans in the test turbines The control turbines were left untreated. |
Oxygenal 6 (H2O2 3% v/v, Ag (+) 0.001% w/v) 10 min |
Mean log load reduction Total viable count – Colony-forming units (CFU) |
DUWLs disinfection with H2O2-Ag (+) minimizes the mycotic load of planktonic pathogens. The mean log load reductions of 6.3 log CFU |
|
Barbot et al38 France |
Laboratory |
C. albicans C. glabrata C. parapsilosis Both planktonic (1 h) and sessile (24-360 h) yeasts |
Chlorine (3-118 μg/mL) H2O2 (700-9000 μg/mL) Oxygenal 6 6% (w/v) of H2O2 and silver ions 15 min |
Total viable count – (CFU) | None of the tested disinfectants could eradicate yeasts. Oxygenal 6 displayed a reduced antimycotic activity against sessile C. albicans and in coculture (yeasts and amoeba). |
However, it appears that sustained infusion of disinfectants for a prolonged period is the most effective decontamination regimen as noted by Szymańska et al28 who noted that 0.25% hydrogen peroxide for 30 minutes, followed by the continuous presence of 0.02% hydrogen peroxide in DUWLs, significantly reduced the overall fungal counts as well as the individual species.
Nevertheless, Porteous et al39 reported that continuous infusion of disinfectants in water lines, as in the case of overnight irrigation, may modify the natural water flora, potentially encouraging the proliferation of fungi, present in small quantities in the municipal water supply systems.
A few workers have evaluated the antimycotic effect on the Dental laboratory Waterlines (DLWL). Costa et al35 conducted a study where they evaluated the effectiveness of Calbenium (composed of EDTA, Benzalkonium hydrochloride, and chloramine T) and Sterispray (70% ethyl alcohol) on C. albicans in polymicrobial biofilms containing Pseudomonas aeruginosa and Vermamoeba vermiformis. They observed that the 0.5% Calbenium and 3%, Sterispray regimen eradicated C. albicans after a 15-minutes. It is worth noting that water stagnation in DUWLs permitted the continuous persistence of fungal cells in biofilms but mitigated their proliferation.
Antimycotic agents appear to be highly effective against the planktonic as opposed to biofilm phase yeasts. Thus Petti and colleagues37 showed the efficacy of H2O2-Ag+(Oxygenal 6) in reducing the fungal burden of planktonic C. albicans from dental turbines with a mean logarithmic reduction of 6.3 log CFU.
On the contrary, Barbot et al38 reported contrasting findings after evaluating several disinfectants. While some agents such as chlorine, H2O2 alone, or H2O2 with silver ions (Oxygenal 6) have shown promising results against planktonic yeasts, their effectiveness against sessile forms in complex microbial ecosystems with eukaryotic amoebae appears to be limited.
Discussion
This review represents the first comprehensive evaluation of fungal contamination in DUWLs across a wide range of dental settings, from hospital clinics to private offices. The wide spectrum of fungal species identified in reviewed reports emanating from different parts of the world underscores the complex and universal challenge of fungal contamination in dental care environments.
Before 1990, dental units differed significantly from modern units, making the older data less applicable to current clinical practices. The significant technological advancements in DUWL design that occurred around this time, including the introduction of antiretraction valves and in-line filtration systems, have substantially impacted biofilm formation and waterline contamination. Due to their unique design, architecture, and operational conditions, DUWLs provide an ideal ecosystem for microbial proliferation. The physical characteristics of DUWLs, such as the interconnected narrow-bore plastic tubes, high surface area to volume ratio, and type of construction material, appear to promote biofilm development.4,19,22 Environmental conditions within the DUWLs, including the ambient low temperature, can further facilitate the proliferation of microbes including fungi that thrive under adverse conditions.8,40 Warm water, especially when it remains stagnant overnight or over the weekend, can support the growth of mould and other microorganisms. In addition, operational routines, such as the intermittent use of dental lines and the laminar flow of water, result in water stagnation, microbial adhesion, and biofilm formation, exacerbating the issue.10,23 Additionally, Hoogenkamp et al highlight biofilms’ complex, multikingdom nature in DUWLs, where bacteria, fungi, and amoebae coexist and interact. The presence of amoebae correlates with higher fungal and bacterial DNA levels, showing that amoebae not only feed on biofilm components but also help protect these microorganisms, including fungi, from external antimicrobials. These insights emphasize that fungi in biofilms cannot be considered in isolation, as their survival appeared to be closely linked to bacterial and amoebal interactions within the biofilm ecosystem.41 Therefore, having a comprehensive understanding of the factors contributing to microbial proliferation in DUWLs is, therefore, crucial in addressing the challenge of fungal contamination.
Our review highlights the wide variety of fungi that can thrive in DUWLs. As shown in 4, these include common pathogenic Candida species, as well as other related yeasts. Additionally, a diverse range of moulds belonging to genera such as Acremonium, Aspergillus, Bipolaris, Citromyces, Fusarium, Penicillium, Rhinocladiella, Sclerotium, Stachybotrys, and Trichoderma was prevalent in DUWLs, and reported in almost all studies reviewed. These moulds encompassed both familiar and more unusual species which are saprophytes, that rarely cause human infections.
Moulds possess unique biological and structural characteristics that contribute to their resilience and incomplete eradication in waterlines. For instance they form a complex and robust biofilm that can firmly adhere to the interior tubing and water reservoirs of DUWLs, making them particularly challenging to eliminate.42 Hydrophobins, small proteins secreted by filamentous fungi play a crucial role in their adhesion to hydrophobic surfaces such as the plastic surfaces of DUWL tubes.43 Also, their filamentous nature allows them to anchor deeply into biofilms, providing a robust defence against disinfectants.
The ability of moulds, as opposed to yeasts, to produce spores and survive adversities including extreme environmental stresses and assaults by disinfectants makes them further resilient.44 There spores can germinate under favourable conditions, initiating contamination cycles that repeatedly compromise the waterline system.45,46 Additionally, moulds exhibit remarkable resilience to environmental pressures, exhibiting a high tolerance to pH and temperature variations and nutrient fluctuations.47 This environmental resilience enables moulds to survive under conditions that typically suppress or eliminate other microorganisms.47,48
While Candida species are indeed known to cause opportunistic infections, the presence of Candida in DUWLs does not imply direct causation. Rather, their presence in dental waterlines represents a potential risk, particularly for immunocompromised patients more susceptible to opportunistic fungal infections. Biofilm-associated Candida may detach and contaminate water output, but infection requires additional predisposing factors, such as a compromised immune system or disrupted oral mucosal barriers. Therefore, the risk of infection from fungi in DUWLs is particularly relevant in the context of vulnerable patient populations.17,18 Routine dental treatments would unlikely regularly expose patients to infectious doses of fungi, especially in healthy individuals. Comparatively, the risk from other common water sources, such as personal drinking water bottles, may also be higher due to prolonged storage and possible fungal proliferation in this population.18
The presence of moulds in DUWLs has significant health implications. Moulds are known to produce mycotoxins and can elicit allergic responses, asthma, and serious pulmonary infections, particularly in immunocompromised individuals.48,49 Thus, to effectively manage mould contamination in DUWLs antimicrobials and disinfectants with greater potency than those used against bacteria and yeasts are needed. Perhaps such heightened resistance warrants the use of higher concentrations of disinfectants. Nevertheless, caution must be exercised to ensure that such measures align with the local, regional, and federal safety standards required for the decontamination of DUWL. Fungi are part of the biofilm matrix and play a well-established role in increasing biofilm complexity.50 Their response to antimycotics can vary depending on the biofilm's overall composition and structure. While essential for controlling microbes, long-term use of high-potency antimicrobials in DUW systems can also increase the risk of mould growth by creating a selection pressure favouring resistant strains and potentially leading to biofilm formation.50 Biofilm disruption inevitably affects fungi and other biofilm components, potentially inhibiting or enhancing fungal growth based on the antimycotic used.41 Therefore, a well-informed and strategic approach is essential for addressing mould contamination in dental care environments, underscoring the critical importance of continuous research and the need to refine disinfection protocols to navigate these complex challenges effectively. As mentioned above, a glaring lapse in the current recommendation and standards for DUWL decontamination primarily focuses on bacterial contamination, while specific guidelines for permissible numbers of yeasts or moulds are notably absent. This can be considered a gap in the existing recommendations, as fungal contaminants in DUWLs can have potential health implications, particularly for immunocompromised individuals.51
Finally, Porteous et al39 noted that waterline treatment, especially with continuous-use waterline cleaners, may inadvertently alter the natural water flora in a way that encourages fungal proliferation. Such unintended consequence of DUWL disinfection emphasizes the delicate balance required in managing DUWLs, where the goal is to eliminate potential pathogens without disrupting the microbial ecosystem in a manner that favours the growth of opportunistic pathogens.
Limitations
A major limitation identified in the reviewed literature is the absence of data, in most studies, on the age of dental units from which water samples were obtained. There is evidence to suggest that both the age of dental units as well as the nature of the dental practice, whether it specializes in particular areas of dentistry can influence the composition of biofilms within DUWLs.52 Furthermore, many of the reviewed studies had a small sample size, with contamination assessments conducted in merely two to three dental units. This limitation obviated a thorough statistical analysis of the results.
Additionally, a number of reviews were quantitative studies and did not analyse in detail the flora cultured, while others focused exclusively on Candida species and ignored the mould components. The identification of the DUWL flora was almost exclusively performed in all studies using traditional plate culture techniques, and only a few studies employed newer fungal identification methods such as Next-Generation Sequencing24 or MALDI-TOF,23,25,26 methodology. Additionally, the variability in detection techniques across studies may influence the outcomes based on the sensitivity and specificity of the methods employed, particularly between culture-based and molecular techniques (eg, quantitative polymerase chain reaction and sequencing). These limitations underscore the necessity for further detailed research into fungal contamination of DUWLs.
Biofilms present a significant challenge in DUWLs due to their inherent resistance to antimicrobial agents and ability to regenerate after initial decontamination. Therefore, preventing fungal regrowth within biofilms is critical to maintaining waterline hygiene. The reviewed literature primarily emphasizes that planktonic cells are susceptible to many antimicrobial agents. Biofilm-associated cells exhibit enhanced resistance due to the protective matrix that encases the microbial community.50 Thus, biofilm-specific prevention strategies are paramount to sustaining fungal contamination control in DUWLs’. Therefore, future studies should address planktonic and sessile microbial forms in waterline contamination control.
Hence, conducting a thorough assessment of fungal biofilm composition within the DUWLs, will facilitate development of standardized and robust treatment protocols to mitigate this potential health risk to both patients and especially dental personnel who are routinely exposed to DUWL water during their professional lifetime. In the absence of specific regulations regarding permissible levels of fungi in DUWLs by dental authorities, our review can provide valuable foundational data for the development of legislation on this matter.
Conclusions
Fungal contamination of DUWLs is a complex, multifaceted issue involving water line design, operational protocols, and the complex nature of biofilm-associated microbial communities. Controlling DUWL contamination poses significant challenges, due to the resilience of biofilms and the diversity of fungal species involved. The review highlights the intricate fungal ecosystems in DUWLs and emphasizes the importance of targeted antimicrobial strategies to maintain waterline hygiene. However, the review indicates that total eradication of fungi could not be achieved despite the application of varying concentrations, types, and frequencies of the disinfectants that are current. This underscores the need for continued research to develop new, optimized treatment protocols specifically tailored to eradicate fungi from DUWLs. Finally, continued research efforts should focus on understanding the mechanisms of fungal persistence and resistance within biofilms, exploring alternative disinfection methods, and investigating the effectiveness of novel antimicrobial agents.
Author contributions
NS, KSF, and LPS performed data collation analysis and manuscript writing; TP, NBR, and MMM critically examined and edited the final versions of the manuscript. All authors approved the final version for publication.
Conflict of interest
The author(s) below of the captioned paper have declared no potential conflicts of interest.
Acknowledgments
Acknowledgements
TP was supported by the Health Systems Research Institute, National Research Council of Thailand (N42A650229), Faculty of Dentistry (DRF68_007), and Thailand Science Research and Innovation Fund Chulalongkorn University (HEA_FF_68_008_3200_001).
Data Availability Statement
Supplementary data files can be provided upon request.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.identj.2024.10.020.
Appendix. Supplementary materials
References
- 1.Walker J.T., Marsh P.D. Microbial biofilm formation in DUWS and their control using disinfectants. J Dent. 2007;35(9):721–730. doi: 10.1016/j.jdent.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Ji X.-Y., Fei C.-N., Zhang Y., Liu J., Liu H., Song J. Three key factors influencing the bacterial contamination of dental unit waterlines: a 6-year survey from 2012 to 2017. Int Dent J. 2019;69(3):192–199. doi: 10.1111/idj.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buitrago J.M., Kolbe R.J., Siqueira M.F. Dental unit waterline testing practices: an 11-Year retrospective study. BMC Oral Health. 2023;23(1):867. doi: 10.1186/s12903-023-03590-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spagnolo A.M., Sartini M., Cristina M.L. Microbial contamination of dental unit waterlines and potential risk of infection: a narrative review. Pathogens. 2020;9(8):1–11. doi: 10.3390/pathogens9080651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marino F., Mazzotta M., Pascale M.R., Derelitto C., Girolamini L., Cristino S. First water safety plan approach applied to a Dental Clinic complex: identification of new risk factors associated with Legionella and P. aeruginosa contamination, using a novel sampling, maintenance and management program. J Oral Microbiol. 2023;15(1):2223477. doi: 10.1080/20002297.2023.2223477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker J.T., Bradshaw D.J., Bennett A.M., Fulford M.R., Martin M.V., Marsh P.D. Microbial biofilm formation and contamination of dental-unit water systems in general dental practice. Appl Environ Microbiol. 2000;66(8):3363–3367. doi: 10.1128/aem.66.8.3363-3367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karpay R.I., Plamondon T.J., Mills S.E., Dove S.B. Combining periodic and continuous sodium hypochlorite treatment to control biofilms in dental unit water systems. J Am Dent Assoc. 1999;130(7):957–965. doi: 10.14219/jada.archive.1999.0336. [DOI] [PubMed] [Google Scholar]
- 8.Barbot V., Robert A., Rodier M.H., Imbert C. Update on infectious risks associated with dental unit waterlines. FEMS Immunol Med Microbiol. 2012;65(2):196–204. doi: 10.1111/j.1574-695X.2012.00971.x. [DOI] [PubMed] [Google Scholar]
- 9.Lizzadro J., Mazzotta M., Girolamini L., Dormi A., Pellati T., Cristino S. Comparison between two types of dental unit waterlines: how evaluation of microbiological contamination can support risk containment. Int J Environ Res Public Health. 2019;16(3):1–14. doi: 10.3390/ijerph16030328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman D.C., O’Donnell M.J., Shore A.C., Russell R.J. Biofilm problems in dental unit water systems and its practical control. J Appl Microbiol. 2009;106(5):1424–1437. doi: 10.1111/j.1365-2672.2008.04100.x. [DOI] [PubMed] [Google Scholar]
- 11.O’Donnell M.J., Boyle M., Swan J., Russell R.J., Coleman D.C. A centralised, automated dental hospital water quality and biofilm management system using neutral Ecasol maintains dental unit waterline output at better than potable quality: a 2-year longitudinal study. J Dent. 2009;37(10):748–762. doi: 10.1016/j.jdent.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell M.J., Shore A.C., Russell R.J., Coleman D.C. Optimisation of the long-term efficacy of dental chair waterline disinfection by the identification and rectification of factors associated with waterline disinfection failure. J Dent. 2007;35(5):438–451. doi: 10.1016/j.jdent.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Samaranayake L., Fakhruddin K.S. Pandemics past, present, and future: their impact on oral health care. J Am Dent Assoc. 2021;152(12):972–980. doi: 10.1016/j.adaj.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douglas L.J. Candida biofilms and their role in infection. Trends Microbiol. 2003;11(1):30–36. doi: 10.1016/s0966-842x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 15.Hernández-Chávez M.J., Pérez-García L.A., Niño-Vega G.A., Mora-Montes H.M. Fungal strategies to evade the host immune recognition. J Fungi (Basel) 2017;3(4):1–28. doi: 10.3390/jof3040051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Donnell M.J., Shore A.C., Coleman D.C. A novel automated waterline cleaning system that facilitates effective and consistent control of microbial biofilm contamination of dental chair unit waterlines: a one-year study. J Dent. 2006;34(9):648–661. doi: 10.1016/j.jdent.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Garvey M., Meade E., Rowan N.J. Effectiveness of front line and emerging fungal disease prevention and control interventions and opportunities to address appropriate eco-sustainable solutions. Sci Total Environ. 2022;851 doi: 10.1016/j.scitotenv.2022.158284. [DOI] [PubMed] [Google Scholar]
- 18.Sonigo, P., A. De Toni, and K. Reilly, A Review Of Fungi In Drinking Water And The Implications For Human Health. Final report. Reference: WD 0906; April 2011. DEFRA—University of Sheffield and University of Bristol, 2011: p. 1–107.
- 19.O’Donnell M.J., Boyle M.A., Russell R.J., Coleman D.C. Management of dental unit waterline biofilms in the 21st century. Future Microbiol. 2011;6(10):1209–1226. doi: 10.2217/fmb.11.104. [DOI] [PubMed] [Google Scholar]
- 20.Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquhoun H., Levac D., et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 21.Bain R., Cronk R., Wright J., Yang H., Slaymaker T., Bartram J. Fecal contamination of drinking-water in low- and middle-income countries: a systematic review and meta-analysis. PLOS Medicine. 2014;11(5) doi: 10.1371/journal.pmed.1001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bayani M., Raisolvaezin K., Almasi-Hashiani A., Mirhoseini S.H. Bacterial biofilm prevalence in dental unit waterlines: a systematic review and meta-analysis. BMC Oral Health. 2023;23(1):158. doi: 10.1186/s12903-023-02885-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Błaszczyk B., Pajaczkowska M, Nowicka J., Szymonowicz M., Zakrzewski W., Lubojański A., et al. Microbiological evaluation of water used in dental units. Water. 2022;14:1–17. doi: 10.3390/w14060915. [DOI] [Google Scholar]
- 24.Fan C., Gu H., Liu L., Zhu H., Yan J., Huo Y. Distinct microbial community of accumulated biofilm in dental unit waterlines of different specialties. Front Cell Infect Microbiol. 2021;11:1–14. doi: 10.3389/fcimb.2021.670211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omran E.A.H., Abbass A.A.G., Abaza A.F., Elzouki E.M. Study of some risk factors for fungal contamination of dental unit waterlines in Alexandria, Egypt. J Infect Dev Ctries. 2021;15(8):1197–1204. doi: 10.3855/jidc.13810. [DOI] [PubMed] [Google Scholar]
- 26.Mazari W., Boucherit-Otmani Z., El Haci I.A., Ilahi A., Boucherit K. Risk assessment for the spread of Candida sp. in dental chair unit waterlines using molecular techniques. Int Dent J. 2018;68(6):386–392. doi: 10.1111/idj.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadaifciler D.G., Ökten S., Sen B. Mycological contamination in dental unit waterlines in Istanbul, Turkey. Braz J Microbiol. 2013;44(3):977–981. doi: 10.1590/s1517-83822013000300049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szymańska J. Evaluation of mycological contamination of dental unit waterlines. Ann Agric Environ Med. 2005;12(1):153–155. [PubMed] [Google Scholar]
- 29.Damasceno J.L., Dos Santos R.A., Barbosa A.H., Casemiro L.A., Pires R.H., Martins C.H.G. Risk of fungal infection to dental patients. Sci World J. 2017;2017:2982478. doi: 10.1155/2017/2982478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samaranayake L.P., MacFarlane T.W. Wright; London: 1990. Oral candidiasis. [Google Scholar]
- 31.Voelz K., May R.C. Cryptococcal interactions with the host immune system. Eukaryot Cell. 2010;9(6):835–846. doi: 10.1128/EC.00039-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chris K., David W.D. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015;70(3):270. doi: 10.1136/thoraxjnl-2014-206291. [DOI] [PubMed] [Google Scholar]
- 33.Hussain Akbar J., Behbehani J., Karched M. Biofilm growth and microbial contamination of dental unit waterlines at Kuwait University dental center. Front Oral Health. 2022;3 doi: 10.3389/froh.2022.1071018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujita M., Mashima I., Nakazawa F. Monitoring the decontamination efficacy of the novel Poseidon-S disinfectant system in dental unit water lines. J Microbiol Immunol Infect. 2017;50(3):270–276. doi: 10.1016/j.jmii.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Costa D., Girardot M., Bertaux J., Verdon J., Imbert C. Efficacy of dental unit waterlines disinfectants on a polymicrobial biofilm. Water Research. 2016;91:38–44. doi: 10.1016/j.watres.2015.12.053. [DOI] [PubMed] [Google Scholar]
- 36.Costa D., Mercier A., Gravouil K., Lesobre J., Verdon J., Imbert C. Occurrence and diversity of both bacterial and fungal communities in dental unit waterlines subjected to disinfectants. Pathog Dis. 2016;74(7):1–11. doi: 10.1093/femspd/ftw094. [DOI] [PubMed] [Google Scholar]
- 37.Petti S., Polimeni A., Allen M.J. Dental unit water treatment with hydrogen peroxide and monovalent silver ions artificially contaminated with freshly isolated pathogens. Ann Ig. 2015;27(6):789–798. doi: 10.7416/ai.2015.2072. [DOI] [PubMed] [Google Scholar]
- 38.Barbot V., Costa D., Deborde M., Imbert C. Efficacy of dental unit disinfectants against Candida spp. and Hartmannella vermiformis. Pathog Dis. 2014;70(3):289–296. doi: 10.1111/2049-632X.12127. [DOI] [PubMed] [Google Scholar]
- 39.Porteous N.B., Redding S.W., Thompson E.H., Grooters A.M., De Hoog S., Sutton DA. Isolation of an unusual fungus in treated dental unit waterlines. J Am Dent Assoc. 2003;134(7):853–858. doi: 10.14219/jada.archive.2003.0283. [DOI] [PubMed] [Google Scholar]
- 40.Calero Preciado C., Boxall J., Soria-Carrasco V., Martínez S., Douterelo I. Implications of climate change: how does increased water temperature influence biofilm and water quality of chlorinated drinking water distribution systems? Front Microbiol. 2021;12:1–15. doi: 10.3389/fmicb.2021.658927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoogenkamp M.A., Brandt B.W., Laheij A.M.G.A., de Soet J.J., Crielaard W. The microbiological load and microbiome of the Dutch dental unit; ‘please, hold your breath’. Water Res. 2021;200:117205. doi: 10.1016/j.watres.2021.117205. [DOI] [PubMed] [Google Scholar]
- 42.Chen P., Zeng J., Hong F., Li C., Wang H., Yu X. The importance of biofilm contamination control for dental unit waterlines: a multicenter assessment of the microbiota diversity of biofilm in dental unit waterlines. J Oral Microbiol. 2024;16(1):2299496. doi: 10.1080/20002297.2023.2299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linder M.B., Szilvay G.R., Nakari-Setälä T., Penttilä M.E. Hydrophobins: the protein-amphiphiles of filamentous fungi. FEMS Microbiol Rev. 2005;29(5):877–896. doi: 10.1016/j.femsre.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Corbu V.M., Gheorghe-Barbu I., Dumbravă A.Ș., Vrâncianu C.O., Șesan T.E. Current insights in fungal importance—a comprehensive review. Microorganisms. 2023;11:1–52. doi: 10.3390/microorganisms11061384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dijksterhuis J. The fungal spore and food spoilage. Curr Opin Food Sci. 2017;17:68–74. [Google Scholar]
- 46.Lin S., Yang L., Chen G., Li B., Chen D., Li L., Xu Z. Pathogenic features and characteristics of food borne pathogens biofilm: biomass, viability and matrix. Microbial Pathogenesis. 2017;111:285–291. doi: 10.1016/j.micpath.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Liu S., Le Mauff F., Sheppard D.C., Zhang S. Filamentous fungal biofilms: conserved and unique aspects of extracellular matrix composition, mechanisms of drug resistance and regulatory networks in Aspergillus fumigatus. NPJ Biofilms Microbiomes. 2022;8(1):83. doi: 10.1038/s41522-022-00347-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hardin B.D., Kelman B.J., Saxon A. Adverse human health effects associated with molds in the indoor environment. J Occup Environ Med. 2003;45(5):470–478. doi: 10.1097/00043764-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Robbins C.A., Swenson L.J., Nealley M.L., Gots R.E., Kelman B.J. Health effects of mycotoxins in indoor air: a critical review. Appl Occup Environ Hyg. 2000;15(10):773–784. doi: 10.1080/10473220050129419. [DOI] [PubMed] [Google Scholar]
- 50.Wang D., Zeng N., Li C., Li Z., Zhang N., Li B. Fungal biofilm formation and its regulatory mechanism. Heliyon. 2024;10(12) doi: 10.1016/j.heliyon.2024.e32766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fakhruddin K.S., Matsubara V.H., Warnakulasuriya S., Tilakaratne W.M., Ngo H.C., Samaranayake L.P. Mucormycosis of the mandible and tongue: a systematic scoping review. Int Dent J. 2024;74(3):454–472. doi: 10.1016/j.identj.2023.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dahlen G. Biofilms in dental unit water lines. Monogr Oral Sci. 2021;29:12–18. doi: 10.1159/000510195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplementary data files can be provided upon request.