Abstract
The biogenesis of iron–sulphur clusters requires the co-ordinated delivery of both iron and sulphur. It is now clear that sulphur in iron–sulphur clusters is derived from L-cysteine by cysteine desulphurases. However, the iron donor for the iron–sulphur cluster assembly still remains elusive. Our previous studies indicated that Escherichia coli IscA, a member of the iron–sulphur cluster assembly machinery, is an iron-binding protein that can provide iron for the iron–sulphur cluster assembly in a proposed scaffold IscU. To determine how the iron centre in IscA is transferred for the iron–sulphur cluster assembly in IscU, we explore the mobility of the iron centre in IscA. The UV–visible and EPR measurements show that L-cysteine, but not IscU, is able to mobilize the iron centre in IscA and make the iron available for the iron–sulphur cluster assembly in IscU. Other related biological thiols such as N-acetyl-L-cysteine or reduced glutathione have no effect on the iron centre of IscA, suggesting that L-cysteine is unique in mobilizing the iron centre of IscA. Nevertheless, L-cysteine alone is not sufficient to transfer the iron from IscA to IscU. Both L-cysteine and cysteine desulphurase (IscS) are required for the IscA-mediated assembly of iron–sulphur clusters in IscU. The results suggest that L-cysteine may have two distinct functions in the biogenesis of iron–sulphur clusters: to mobilize the iron centre in IscA and to provide sulphur via cysteine desulphurase (IscS) for the iron–sulphur cluster assembly in IscU.
Keywords: cysteine desulphurase, L-cysteine, iron-binding protein, iron–sulphur cluster, IscA, IscU
INTRODUCTION
Through evolution, iron–sulphur clusters have become integral parts of diverse biological processes such as energy conversion, the citric acid cycle, nitrogen fixation, amino acid biosynthesis, haem and biotin biosynthesis, DNA synthesis and DNA repair, and regulation of gene expression [1,2]. However, the biogenesis of iron–sulphur clusters is still not fully understood. The pioneering work by Zheng et al. [3] revealed that two proteins, NifS and NifU, are essential for the iron–sulphur cluster assembly in nitrogenase in Azotobacter vinelandii. NifS, a pyridoxal 5′-phosphate-containing cysteine desulphurase, catalyses cysteine desulphurization and delivers sulphur for the iron–sulphur cluster assembly in NifU [4–6]. NifU is considered to be a scaffold [6] and it eventually transfers the assembled iron–sulphur clusters to nitrogenase through an as yet unknown mechanism [7].
In Escherichia coli, there are three NifS homologues: IscS, SufS and CSD (cysteine sulphinate desulphinase) [8,9]. IscS is a member of the gene cluster iscSUA-hscBA-fdx that is primarily responsible for the biogenesis of iron–sulphur clusters in bacteria [10,11]. Like NifS, IscS catalyses cysteine desulphurization and provides sulphur for the iron–sulphur cluster assembly in IscU, a proposed scaffold that is homologous with the N-terminal domain of NifU [12–16]. Deletion of the iscS gene in E. coli dramatically decreases the specific activities of iron–sulphur proteins [17], suggesting that IscS is the major cysteine desulphurase activity for the biogenesis of iron–sulphur clusters. The residual cysteine desulphurase activity in the IscS-inactivated mutant is apparently provided by SufS, the second NifS homologue in E. coli [18]. SufS is a member of another gene cluster sufABCDSE that represents a redundant activity for the biogenesis of iron–sulphur clusters in bacteria [18–22]. The expression of the gene cluster sufABCDSE is stimulated by cellular oxidative stresses [22,23] and iron starvation [24], implying that the products of the gene cluster sufABCDSE are probably involved in repairing the damaged iron–sulphur clusters in cells.
Whereas sulphur in iron–sulphur clusters is most probably provided by L-cysteine via cysteine desulphurases, the iron donor for iron–sulphur clusters largely remains elusive. The intracellular ‘free’ iron concentration is tightly controlled, because the elevated levels of the ‘free’ iron content promote the production of hydroxyl free radicals via the Fenton reaction [25]. It is thus postulated that specific proteins must be involved in delivering iron for the iron–sulphur cluster assembly in cells. In searching for the iron donor for the iron–sulphur cluster assembly, we found that E. coli IscA, the third gene in the gene cluster iscSUA-hscBA-fdx [10,11], is a novel iron-binding protein with an iron association constant of 3.0×1019 M−1 [26], and that the iron-loaded IscA can provide iron for the iron–sulphur cluster assembly in IscU even under the limited accessible ‘free’ iron conditions [27]. The results suggest that IscA may act as an iron donor for the iron–sulphur cluster assembly in cells.
IscA and its homologues from other organisms were previously characterized as an alternative scaffold for the iron–sulphur cluster assembly, because purified IscA, like IscU, can host transient iron–sulphur clusters and transfer the assembled clusters to target proteins in vitro [28–36]. However, the X-ray crystallographic studies of IscA [37,38] and NMR studies of IscU [39,40] indicate that IscA and IscU are structurally very different proteins. IscA has a stable N-terminal domain and a flexible C-terminal region [37,38]. The protein exists as a tetramer with a central channel formed by the association of the monomers. Within the central channel, the three conserved cysteine residues (Cys-35, -99 and -101) from each IscA monomer are projected to form a ‘cysteine pocket’ [26,37,38]. None of these features are found in IscU [39,40]. The biochemical studies show that IscA and IscU have very different iron-binding activities. Whereas IscA binds iron with an iron association constant of 3.0×1019 M−1, IscU fails to do so under the same experimental conditions [27]. The recent genetic studies further suggest that IscA cannot substitute for IscU in the biogenesis of iron–sulphur clusters in A. vinelandii, as the iscU gene is essential for the bacteria to survive under the defined growth conditions [41]. Collectively, these observations suggest that IscA may have its unique functions in addition to a possible scaffold role in the biogenesis of iron–sulphur clusters. Considering the remarkable iron-binding activity of IscA [26] and its ability to provide iron for the iron–sulphur cluster assembly in IscU [27], we propose that IscA may be responsible for recruiting intracellular ‘free’ iron and delivering the iron for the iron–sulphur cluster assembly in IscU [26,27].
The proposed model requires that IscA transfer the iron centre to IscU for the iron–sulphur cluster assembly via a controlled mechanism. However, because IscU has a much weaker ironbinding activity than IscA [12,27,42], it would be thermodynamically unfavourable for IscA to transfer directly the iron centre to IscU. In the present study, we report that L-cysteine, but not IscU, can mobilize the iron centre in IscA and make the iron available for the iron–sulphur cluster assembly in IscU. The role of L-cysteine in the biogenesis of iron–sulphur clusters will be discussed.
EXPERIMENTAL
Protein purification
Recombinant E. coli IscA [26], IscU [26] and IscS [43] were prepared as described previously. The purity of purified proteins was greater than 95% as judged by electrophoresis analysis on a 15% polyacrylamide gel containing SDS followed by staining with Coomassie Blue. The concentration of apo-IscA was determined using a molar absorbance coefficient ϵ at 260 nm of 2.4 mM−1·cm−1 [26]. The concentrations of IscU and IscS were determined using ϵ at 280 nm of 11.2 and 39.7 mM−1·cm−1 respectively [26]. All protein concentrations mentioned in this paper refer to the monomeric species. The iron-loaded IscA was prepared by incubating apo-IscA with ferrous iron in the presence of dithiothreitol (2 mM) anaerobically at room temperature (24 °C) for 15 min, followed by desalting using a HiTrap desalting column [26]. Alternatively, the thioredoxin and thioredoxin reductase system (50 μM thioredoxin, 2 μM thioredoxin reductase and 2 mM NADPH) was used instead of dithiothreitol for the reconstitution of iron in IscA. Purified desulphoredoxin from the sulphate reducing bacterium Desulfovibrio gigas [44] was a gift from Dr F. Rusnak's group (Mayo Clinic, Rochester, MN, U.S.A.). All protein samples were dissolved in a buffer containing 200 mM NaCl and 20 mM Tris (pH 8.0).
Measurements of ‘free’ iron in solution
The ‘free’ iron released from proteins was measured using an iron indicator ferrozine [3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazene-4,4′-disulphonic acid] [45]. ϵ at 562 nm of 27.9 mM−1·cm−1 for the ferrozine–iron complex was used to calculate the amounts of iron in solutions.
EPR measurements
The EPR spectra of the IscA samples were recorded at X-band on a Bruker ESP-300 spectrometer using an Oxford Instruments ESR-9 flow cryostat (Chemistry Department, Louisiana State University). The EPR conditions are: microwave frequency, 9.45 GHz; microwave power, 10 mW; modulation frequency, 100 kHz; modulation amplitude, 2.0 mT; sample temperature, 4.5 K; receive gain, 1.0×105.
Iron–sulphur cluster assembly in IscU
Purified IscU (100 μM) was incubated with the iron-loaded IscA (150 μM), dithiothreitol (2 mM), NaCl (200 mM), and Tris (20 mM; pH 8.0). The reaction mixture was purged with pure argon gas and preincubated at 37 °C for 5 min before L-cysteine (1 mM) and/or IscS (1 μM) were added to initiate the reaction under anaerobic conditions. The iron–sulphur cluster assembly reactions were monitored in a Beckman DU-640 UV–visible absorption spectrometer equipped with a temperature controller. The amounts of the iron–sulphur clusters assembled in IscU were calculated from the amplitude of the absorption at 456 nm using ϵ of 5.8 mM−1·cm−1 [12].
Repurification of IscA and IscU from reaction solutions
A Mono-Q column (0.98 ml; Amersham Biosciences, Piscataway, NJ, U.S.A.) attached to the FPLC system (Amersham Biosciences) was used for repurification of IscA and IscU from the incubation solutions as described previously [27]. Briefly, samples were loaded on to the Mono-Q column pre-equilibrated with a buffer containing 20 mM Tris (pH 8.0), and eluted with a linear gradient of NaCl (0–1 M) within 10 column volumes at a flow rate of 1 ml·min−1. All solutions were purged with pure argon gas before use. Each eluted fraction was immediately transferred to an anaerobic cuvette and analysed in a Beckman DU-640 UV–visible absorption spectrometer. The eluted samples were then subjected to SDS/PAGE analysis. Under the experimental conditions, IscA and IscU were mainly eluted from the Mono-Q column in fractions 8 and 10 respectively [27].
RESULTS
L-Cysteine is able to mobilize the iron centre in IscA
Our previous studies showed that E. coli IscA is a novel iron-binding protein, and that the iron-loaded IscA can provide iron for the iron–sulphur cluster assembly in IscU in vitro [26,27]. Evidently, the otherwise stable iron centre in IscA must be mobilized and transferred to the iron–sulphur cluster assembly in IscU. However, the remarkable iron-binding activity of IscA [27] implies that IscA cannot directly transfer the iron for the iron–sulphur cluster assembly in IscU, a relatively weaker iron-binding protein [12,27,42]. Indeed, incubation of the iron-loaded IscA with apo-IscU at 37 °C for 20 min anaerobically had no effect on the iron centre in IscA (results not shown). The question as to how the iron centre in IscA is mobilized and transferred for the iron–sulphur cluster assembly in IscU is the subject of the present study.
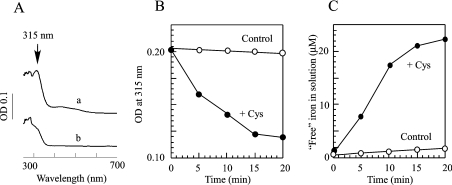
Since iron is probably bound to IscA via cysteine side chains [26,37,38], we speculated that the iron centre in IscA may be mobilized by ‘free’ L-cysteine via ligand exchanging. To test this idea, we incubated the iron-loaded IscA with or without L-cysteine anaerobically at 37 °C for 20 min. Figure 1(A) shows that when the iron-loaded IscA was incubated alone, the absorption peak at 315 nm of IscA, an indication of iron binding in the protein [26], remained unchanged. This is consistent with the previous report that the iron centre in IscA is stable [26]. When the iron-loaded IscA was incubated with L-cysteine, the absorption peak at 315 nm of the iron-loaded IscA was quickly eliminated (Figure 1A). The half-life of the absorption peak at 315 nm of the iron-loaded IscA (50 μM) in the presence of L-cysteine (1 mM) was approx. 5 min (Figure 1B).
Figure 1. L-Cysteine mobilizes the iron centre in IscA.
(A) UV–visible absorption spectra of IscA. The iron-loaded IscA (50 μM) was incubated with Tris (20 mM; pH 8.0), NaCl (200 mM) and dithiothreitol (2 mM) anaerobically (a) or with the addition of L-cysteine (1 mM) (b) at 37 °C for 20 min. After incubation, IscA samples were repurified by passing through a HiTrap desalting column (5 ml). (B) L-cysteine-mediated decay kinetics of the absorption peak at 315 nm of the iron-loaded IscA. Iron-loaded IscA (50 μM) was incubated with (●) or without (○) L-cysteine (1 mM) at 37 °C. The absorptions at 315 nm were measured at each time point indicated. (C) Kinetics of the iron release from IscA by L-cysteine. The iron-loaded IscA (50 μM) was incubated with an iron indicator ferrozine (100 μM) with (+Cys) or without (control) L-cysteine (1 mM) at 37 °C. The amplitude of the absorption peak at 562 nm of the ferrozine–iron complex was used to calculate the amounts of ‘free’ iron in solutions [45]. The experiments were repeated three times and similar results were obtained.
To determine whether the iron centre in IscA was indeed mobilized by L-cysteine, an iron indicator ferrozine [45] was included in the incubation solutions. As shown in Figure 1(C), when the iron-loaded IscA (50 μM) was incubated alone, less than 2 μM iron was released from IscA after incubation. On the other hand, when the iron-loaded IscA (50 μM) was incubated with L-cysteine (1 mM), approx. 22 μM ‘free’ iron was released from the protein. Since the ratio of iron to IscA monomer in the iron-loaded IscA is approx. 0.5 [26], nearly all iron in the iron-loaded IscA was released after incubation with L-cysteine.
To examine whether the mobilization of the iron centre in IscA by L-cysteine is unique, we utilized desulphoredoxin, a well-characterized mononuclear iron protein from D. gigas [44], as a control. Desulphoredoxin binds one mononuclear iron centre via four cysteine residues per polypeptide chain. Like IscA, the iron centre in desulphoredoxin can be readily observed from its characteristic UV–visible absorption spectrum [44]. After incubation with L-cysteine at 37 °C for 20 min, the absorption spectrum of desulphoredoxin remained unchanged, and no iron was released from the protein by L-cysteine (results not shown). These results indicate that the mobilization of the iron centre in IscA by L-cysteine could be specific.
EPR characterization of the L-cysteine-mediated mobilization of the iron centre in IscA
The L-cysteine-mediated mobilization of the iron centre in IscA was further examined using EPR spectroscopy. As reported previously, the iron-loaded IscA has an unusual EPR signal at g=4–6, reflecting a high spin S=3/2 ferric iron centre in the protein [26]. The same EPR signal at g=4–6 was observed in purified IscA [26], indicating that the EPR signal is not an artifact of the iron reconstitution in IscA. The IscA mutants that fail to bind iron did not have any EPR signal at g=4–6, further suggesting that the EPR signal at g=4–6 reflects the iron centre in IscA.
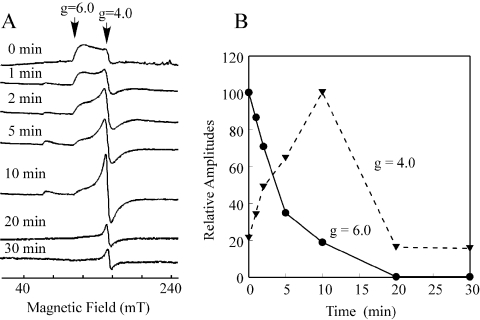
If L-cysteine mobilizes the iron centre in IscA, it is conceivable that the EPR signal at g=4–6 of the iron-loaded IscA will be altered. Indeed, as shown in Figure 2(A), when the iron-loaded IscA was incubated with L-cysteine, the EPR signal at g=4–6 of the iron-loaded IscA was diminished with a half-life of approx. 4 min (Figure 2B). The decay kinetics of the EPR signal at g=4–6 of the iron-loaded IscA was similar to that of the absorption peak at 315 nm of the protein when incubated with L-cysteine (Figure 1B).
Figure 2. EPR characterization of the L-cysteine-mediated mobilization of the iron centre in IscA.
The iron-loaded IscA (600 μM) was incubated with Tris (20 mM; pH 8.0), NaCl (200 mM), dithiothreitol (2 mM) and L-cysteine (1 mM) at 37 °C. At each time point, aliquots (400 μl) were taken and immediately frozen in liquid nitrogen. (A) The EPR spectra of the iron-loaded IscA after incubation with L-cysteine for various time points. The EPR signal at g=4–6 reflects a high spin S=3/2 ferric iron centre in IscA [26]. The EPR signal at g=4.0 represents the ‘free’ ferric iron in solution. All spectra were presented in the same scale. (B) Kinetics of the L-cysteine-mediated mobilization of the iron centre in IscA. Data were obtained from (A). ——, Amplitudes of the EPR signal at g=4–6 of the iron-loaded IscA; and ······, amplitudes of the EPR signal at g=4.0 of the ‘free’ ferric iron in the solution. Results are representative of three independent experiments.
Significantly, as the EPR signal at g=4–6 of the iron-loaded IscA was decreased, a new EPR signal at g=4.0, a signal reflecting the ‘junk’ ferric iron in solution [46], gradually appeared (Figure 2A). The transition of the EPR signal from g=4–6 to g=4.0 clearly indicates that the iron centre of IscA is mobilized by L-cysteine and eventually released to aqueous solution. Nevertheless, the EPR signal at g=4.0 of the ‘junk’ ferric iron faded away after an additional 10 min of incubation (Figure 2A), probably because the released ferric iron was reduced to the EPR-silent ferrous iron by thiols in the solution.
L-Cysteine is unique in mobilizing the iron centre in IscA
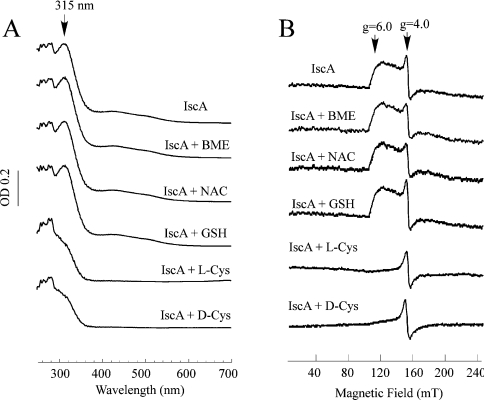
It is possible that other biological thiols can also mobilize the iron centre in IscA. To test this idea, the iron-loaded IscA was incubated with the cysteine-related thiols anaerobically at 37 °C for 20 min. Figure 3(A) shows that unlike L-cysteine, incubation with 2-mercaptoethanol, N-acetyl-L-cysteine, or reduced glutathione had no effect on the absorption peak at 315 nm of the iron-loaded IscA. Similarly, no absorption change at 315 nm of the iron-loaded IscA was observed after incubation with L-serine, L-methionine, or L-alanine (results not shown).
Figure 3. L-Cysteine is unique in mobilizing the iron centre in IscA.
(A) The iron-loaded IscA (100 μM) was incubated with Tris (20 mM; pH 8.0), NaCl (200 mM), dithiothreitol (2 mM) and 1 mM each of 2-mercaptoethanol (BME), N-acetyl-L-cysteine (NAC), glutathione (GSH), L-cysteine (L-Cys) and D-cysteine (D-Cys) respectively at 37 °C for 20 min. The samples were then desalted using a HiTrap desalting column and subjected to UV–visible absorption measurements. (B) The EPR spectra of the same samples as prepared for (A) except that the proteins concentration was approx. 400 μM.
In parallel experiments, the EPR samples were prepared after the iron-loaded IscA was incubated with the cysteine-related thiols anaerobically at 37 °C for 20 min. As shown in Figure 3(B), unlike L-cysteine, 2-mercaptoethanol, N-acetyl-L-cysteine or reduced glutathione failed to change the EPR signal at g=4–6 of the iron-loaded IscA. Collectively, the results show that L-cysteine is unique in mobilizing the iron centre of IscA.
To examine if the stereo structure of cysteine is critical in mobilizing the iron centre in IscA, we incubated the iron-loaded IscA with D-cysteine at 37 °C for 20 min. As shown in Figures 3(A) and 3(B), the absorption peak at 315 nm and the EPR signal at g=4–6 of the iron-loaded IscA were completely eliminated after incubation with D-cysteine. Thus the redox property, not the stereo structure, of L-cysteine is responsible for mobilizing the iron centre in IscA.
Iron transfer from IscA for the iron–sulphur cluster assembly in IscU requires both L-cysteine and cysteine desulphurase (IscS)
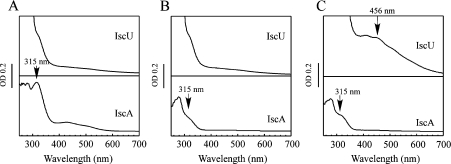
Since the iron-loaded IscA can provide iron for the iron–sulphur cluster assembly in IscU [27], the iron centre in IscA must be transferred to IscU for the iron–sulphur cluster assembly. To explore the possible iron transfer mechanism, the iron-loaded IscA was incubated with apo-IscU anaerobically at 37 °C for 30 min, followed by repurification of IscA and IscU using a Mono-Q column as described in the Experimental section. Figure 4(A) shows that when the iron-loaded IscA was incubated with apo-IscU, the absorption peak at 315 nm of the iron-loaded IscA remained unchanged, suggesting that IscU cannot directly acquire the iron from the iron-loaded IscA under the experimental conditions.
Figure 4. Iron transfer from the iron-loaded IscA to IscU requires both L-cysteine and IscS.
The iron-loaded IscA (150 μM) and apo-IscU (100 μM) were incubated in a buffer containing Tris (20 mM; pH 8.0), NaCl (200 mM) and dithiothreitol (2 mM) anaerobically at 37 °C for 30 min, followed by repurification using a Mono-Q column as described in the Experimental section. (A) UV–visible absorption spectra of repurified IscU (upper trace) and IscA (bottom trace). (B) Same as in (A) except that L-cysteine (1 mM) was included in the incubation solution. (C) Same as in (A), except that both L-cysteine (1 mM) and IscS (1 μM) were included in the incubation solution. The absorption peak at 456 nm of IscU reflects the assembly of [2Fe-2S] clusters. The experiments were repeated three times and similar results were obtained. Abbreviation: OD, absorbance.
When the iron-loaded IscA was incubated with apo-IscU and L-cysteine anaerobically at 37 °C for 30 min, the absorption peak at 315 nm of the iron-loaded IscA was completely removed (Figure 4B). However, no absorption changes were observed in the repurified IscU (Figure 4B), indicating that L-cysteine alone is not sufficient to transfer iron from IscA to IscU. When the iron-loaded IscA and apo-IscU were incubated with L-cysteine and cysteine desulphurase (IscS) anaerobically at 37 °C for 30 min, the absorption peak at 315 nm of the iron-loaded IscA disappeared with the concurrent appearance of an absorption peak at 456 nm of the repurified IscU (Figure 4C), an indication of the assembled [2Fe-2S] clusters in the protein [12]. Using ϵ at 456 nm of 5.8 mM−1·cm−1 for the IscU [2Fe-2S] clusters [12], we estimated that approx. 30–40% of apo-IscU was converted into the [2Fe-2S] cluster-bound IscU. Thus, whereas L-cysteine can mobilize the iron centre in IscA, both L-cysteine and cysteine desulphurase are required to transfer the iron from IscA for the iron–sulphur cluster assembly in IscU.
DISCUSSION
IscA is a key member of the iron–sulphur cluster assembly machinery [10,11], and is highly conserved from bacteria [28–33] to eukaryotic organisms [34–36,47–49]. Previously, E. coli IscA [28] and its homologues from other organisms [28–36] were characterized as an alternative scaffold for the iron–sulphur cluster assembly, because purified IscA, like IscU, can host transient iron–sulphur clusters and transfer the assembled clusters to target proteins in vitro. However, increasing evidence suggests that IscA may have additional roles in the biogenesis of iron–sulphur clusters. First, it has been shown that IscA [37,38] and IscU [39,40] are structurally very different proteins. The striking difference between IscA and IscU is also evident in their iron-binding activity in vitro [26,27]. Whereas IscA binds iron with an iron association constant of 3.0×1019 M−1, IscU fails to do so under the same experimental conditions [26]. Furthermore, the genetic studies show that IscA cannot substitute for IscU in the biogenesis of iron–sulphur clusters in A. vinelandii, because deletion of the iscU gene is lethal to the organism [41]. Although we could not exclude the possibility that IscA may act as an alternative scaffold, the remarkable iron-binding activity of IscA [26] and the ease with which the iron from the iron-loaded IscA is mobilized by L-cysteine (the present study) strongly support the notion that the primary function of IscA is to recruit intracellular ‘free’ iron and deliver the iron for the iron–sulphur cluster assembly in IscU.
The mechanism by which the iron centre in IscA is mobilized by L-cysteine may only be speculated upon at present. The observed transition of the EPR signal at g=4–6 of the iron-loaded IscA to the EPR signal at g=4.0 of the ‘junk’ ferric iron in solution (Figure 2) clearly indicates that the iron centre in IscA is mobilized by L-cysteine and consequently released. It is significant that L-cysteine, but not the cysteine-related compounds such as 2-mercaptoethanol, N-acetyl-L-cysteine, or reduced glutathione, can mobilize the iron centre in IscA (Figure 3). The reason for this unique activity of L-cysteine remains unclear. The redox midpoint potentials of cysteine and glutathione are −250 and −264 mV at pH 7.4 respectively [50], and the pKa values of cysteine (8.3) and glutathione (8.6) are also close to each other. Evidently, these differences could not account for the observed activity of L-cysteine in mobilizing the iron centre of IscA. It has been speculated that L-cysteine may be able to co-ordinate iron with both sulphhydryl and carboxylate groups [51], and that the interaction of sulphur, iron and oxygen atoms in a three-centre π system may promote facile electron transfer [52]. Apparently, the additional carboxy group in glutathione or acetyl group in N-acetyl-L-cysteine may disturb the geometry of the cysteine–iron complex and impact its ability to mobilize the iron centre in IscA. On the other hand, L-cysteine cannot release the iron from desulphoredoxin, a mononuclear iron-binding protein [44], under the same experimental conditions, suggesting that the mobilization of the iron centre in IscA by L-cysteine could be a specific reaction. In this context, we propose that the unique structure of L-cysteine may allow access to the iron centre of IscA, thereby mobilizing the iron centre of the protein.
Although L-cysteine can mobilize the iron centre in IscA (Figures 1 and 2), L-cysteine alone is apparently not sufficient to transfer the iron from IscA to IscU (Figure 4B). Only if both L-cysteine and IscS are present, is the iron in IscA delivered efficiently for the iron–sulphur cluster assembly in IscU (Figure 4C). At least two models may be considered for the mechanism of iron transfer from IscA for the iron–sulphur cluster assembly in IscU. In the first model, cysteine desulphurase (IscS) may catalyse cysteine desulphurization and provide sulphur for the assembly of transient iron–sulphur clusters in IscA, followed by transfer of the assembled clusters to IscU. However, it has been reported that IscA is unable to transfer the assembled iron–sulphur clusters to IscU in the time-scale of the experiments in vitro [26,33]. In the second model, L-cysteine may initially mobilize the iron centre of IscA to form a transient iron–L-cysteine compound. The iron–L-cysteine compound will then be used as the substrate for cysteine desulphurase (IscS) to generate S-Fe elements. Since IscS can transfer sulphane sulphur to IscU via specific protein–protein interactions [14–16], the S-Fe elements in IscS may be directly transferred to IscU in a similar manner. This later model is consistent with the observations that L-cysteine can mobilize the iron centre in IscA (Figures 1 and 2) and that almost all iron in IscA is transferred for iron–sulphur cluster assembly in IscU after incubation with L-cysteine and IscS (Figure 4C). In this model, we propose that L-cysteine has two distinct functions: (i) to mobilize the iron centre in IscA, and (ii) to provide sulphur via cysteine desulphurase (IscS) for iron–sulphur cluster assembly in IscU.
The unique activity of L-cysteine to mobilize the iron centre in IscA may also have important physiological implications. If all biological thiols could mobilize the iron centre in IscA, the iron centre in IscA would be unstable because of abundant small molecular thiols such as glutathione in cells [53]. It appears that the intracellular L-cysteine concentration is tightly controlled because the elevated levels of intracellular L-cysteine content promote the Fenton reaction [54]. The exact intracellular L-cysteine concentration in bacteria or in eukaryotic cells is still currently unknown. Nevertheless, considering the potential roles of L-cysteine in mobilizing the iron centre in IscA and in providing sulphur for the iron–sulphur cluster assembly in IscU, it may be envisioned that the dynamic change of the intracellular L-cysteine content will have an important regulatory role in the biogenesis of iron–sulphur clusters in cells.
Acknowledgments
We thank Dr B. Hales (Chemistry Department, Louisiana State University) for insightful comments on the EPR spectra. This work was supported in part by the American Heart Association and the National Science Foundation (MCB-0416537; to H.D.).
References
- 1.Beinert H., Holm R. H., Munck E. Iron-sulfur clusters: Nature's modular, multipurpose structures. Science. 1997;277:653–659. doi: 10.1126/science.277.5326.653. [DOI] [PubMed] [Google Scholar]
- 2.Frazzon J., Dean D. R. Formation of iron-sulfur clusters in bacteria: an emerging field in bioinorganic chemistry. Curr. Opin. Chem. Biol. 2003;7:166–173. doi: 10.1016/s1367-5931(03)00021-8. [DOI] [PubMed] [Google Scholar]
- 3.Zheng L., White R. H., Cash V. L., Jack R. F., Dean D. R. Cysteine desulfurase activity indicates a role for NIFS in metallocluster biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 1993;90:2754–2758. doi: 10.1073/pnas.90.7.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng L., Dean D. R. Catalytic formation of a nitrogenase iron-sulfur cluster. J. Biol. Chem. 1994;269:18723–18726. [PubMed] [Google Scholar]
- 5.Zheng L., White R. H., Cash V. L., Dean D. R. Mechanism for the desulfurization of L-cysteine catalyzed by the nifS gene product. Biochemistry. 1994;33:4714–4720. doi: 10.1021/bi00181a031. [DOI] [PubMed] [Google Scholar]
- 6.Yuvaniyama P., Agar J. N., Cash V. L., Johnson M. K., Dean D. R. NifS-directed assembly of a transient [2Fe-2S] cluster within the NifU protein. Proc. Natl. Acad. Sci. U.S.A. 2000;97:599–604. doi: 10.1073/pnas.97.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dos Santos P. C., Smith A. D., Frazzon J., Cash V. L., Johnson M. K., Dean D. R. Iron-sulfur cluster assembly: NifU-directed activation of the nitrogenase Fe protein. J. Biol. Chem. 2004;279:19705–19711. doi: 10.1074/jbc.M400278200. [DOI] [PubMed] [Google Scholar]
- 8.Flint D. H. Escherichia coli contains a protein that is homologous in function and N-terminal sequence to the protein encoded by the nifS gene of Azotobacter vinelandii and that can participate in the synthesis of the Fe-S cluster of dihydroxy-acid dehydratase. J. Biol. Chem. 1996;271:16068–16074. [PubMed] [Google Scholar]
- 9.Mihara H., Kurihara T., Yoshimura T., Esaki N. Kinetic and mutational studies of three NifS homologs from Escherichia coli: mechanistic difference between L-cysteine desulfurase and L-selenocysteine lyase reactions. J. Biochem. (Tokyo) 2000;127:559–567. doi: 10.1093/oxfordjournals.jbchem.a022641. [DOI] [PubMed] [Google Scholar]
- 10.Zheng L., Cash V. L., Flint D. H., Dean D. R. Assembly of iron-sulfur clusters. Identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii. J. Biol. Chem. 1998;273:13264–13272. doi: 10.1074/jbc.273.21.13264. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi Y., Nakamura M. Functional assignment of the ORF2-iscS-iscU-iscA-hscB-hscA-fdx-ORF3 gene cluster involved in the assembly of Fe-S clusters in Escherichia coli. J. Biochem. (Tokyo) 1999;126:917–926. doi: 10.1093/oxfordjournals.jbchem.a022535. [DOI] [PubMed] [Google Scholar]
- 12.Agar J. N., Krebs C., Frazzon J., Huynh B. H., Dean D. R., Johnson M. K. IscU as a scaffold for iron-sulfur cluster biosynthesis: sequential assembly of [2Fe-2S] and [4Fe-4S] clusters in IscU. Biochemistry. 2000;39:7856–7862. doi: 10.1021/bi000931n. [DOI] [PubMed] [Google Scholar]
- 13.Agar J. N., Yuvaniyama P., Jack R. F., Cash V. L., Smith A. D., Dean D. R., Johnson M. K. Modular organization and identification of a mononuclear iron-binding site within the NifU protein. J. Biol. Inorg. Chem. 2000;5:167–177. doi: 10.1007/s007750050361. [DOI] [PubMed] [Google Scholar]
- 14.Urbina H. D., Silberg J. J., Hoff K. G., Vickery L. E. Transfer of sulfur from IscS to IscU during Fe/S cluster assembly. J. Biol. Chem. 2001;276:44521–44526. doi: 10.1074/jbc.M106907200. [DOI] [PubMed] [Google Scholar]
- 15.Smith A. D., Agar J. N., Johnson K. A., Frazzon J., Amster I. J., Dean D. R., Johnson M. K. Sulfur transfer from IscS to IscU: the first step in iron-sulfur cluster biosynthesis. J. Am. Chem. Soc. 2001;123:11103–11104. doi: 10.1021/ja016757n. [DOI] [PubMed] [Google Scholar]
- 16.Kato S., Mihara H., Kurihara T., Takahashi Y., Tokumoto U., Yoshimura T., Esaki N. Cys-328 of IscS and Cys-63 of IscU are the sites of disulfide bridge formation in a covalently bound IscS/IscU complex: implications for the mechanism of iron-sulfur cluster assembly. Proc. Natl. Acad. Sci. U.S.A. 2002;99:5948–5952. doi: 10.1073/pnas.082123599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz C. J., Djaman O., Imlay J. A., Kiley P. J. The cysteine desulfurase, IscS, has a major role in in vivo Fe-S cluster formation in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9009–9014. doi: 10.1073/pnas.160261497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi Y., Tokumoto U. A third bacterial system for the assembly of iron-sulfur clusters with homologs in archaea and plastids. J. Biol. Chem. 2002;277:28380–28383. doi: 10.1074/jbc.C200365200. [DOI] [PubMed] [Google Scholar]
- 19.Patzer S. I., Hantke K. SufS is a NifS-like protein, and SufD is necessary for stability of the [2Fe-2S] FhuF protein in Escherichia coli. J. Bacteriol. 1999;181:3307–3309. doi: 10.1128/jb.181.10.3307-3309.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nachin L., Loiseau L., Expert D., Barras F. SufC: an unorthodox cytoplasmic ABC/ATPase required for [Fe-S] biogenesis under oxidative stress. EMBO J. 2003;22:427–437. doi: 10.1093/emboj/cdg061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurihara T., Mihara H., Kato S., Yoshimura T., Esaki N. Assembly of iron-sulfur clusters mediated by cysteine desulfurases, IscS, CsdB and CSD, from Escherichia coli. Biochim. Biophys. Acta. 2003;1647:303–309. doi: 10.1016/s1570-9639(03)00078-5. [DOI] [PubMed] [Google Scholar]
- 22.Zheng M., Wang X., Templeton L. J., Smulski D. R., LaRossa R. A., Storz G. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J. Bacteriol. 2001;183:4562–4570. doi: 10.1128/JB.183.15.4562-4570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J. H., Yeo W. S., Roe J. H. Induction of the sufA operon encoding Fe-S assembly proteins by superoxide generators and hydrogen peroxide: involvement of OxyR, IHF and an unidentified oxidant-responsive factor. Mol. Microbiol. 2004;51:1745–1755. doi: 10.1111/j.1365-2958.2003.03946.x. [DOI] [PubMed] [Google Scholar]
- 24.Outten F. W., Djaman O., Storz G. A suf operon requirement for Fe-S cluster assembly during iron starvation in Escherichia coli. Mol. Microbiol. 2004;52:861–872. doi: 10.1111/j.1365-2958.2004.04025.x. [DOI] [PubMed] [Google Scholar]
- 25.Keyer K., Gort A. S., Imlay J. A. Superoxide and the production of oxidative DNA damage. J. Bacteriol. 1995;177:6782–6790. doi: 10.1128/jb.177.23.6782-6790.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding H., Clark R. J. Characterization of iron binding in IscA, an ancient iron-sulphur cluster assembly protein. Biochem. J. 2004;379:433–440. doi: 10.1042/BJ20031702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding H., Clark R. J., Ding B. IscA mediates iron delivery for assembly of iron-sulfur clusters in IscU under the limited accessible free iron conditions. J. Biol. Chem. 2004;279:37499–37504. doi: 10.1074/jbc.M404533200. [DOI] [PubMed] [Google Scholar]
- 28.Ollagnier-de-Choudens S., Mattioli T., Takahashi Y., Fontecave M. Iron-sulfur cluster assembly: characterization of IscA and evidence for a specific and functional complex with ferredoxin. J. Biol. Chem. 2001;276:22604–22607. doi: 10.1074/jbc.M102902200. [DOI] [PubMed] [Google Scholar]
- 29.Krebs C., Agar J. N., Smith A. D., Frazzon J., Dean D. R., Huynh B. H., Johnson M. K. IscA, an alternate scaffold for Fe-S cluster biosynthesis. Biochemistry. 2001;40:14069–14080. doi: 10.1021/bi015656z. [DOI] [PubMed] [Google Scholar]
- 30.Wollenberg M., Berndt C., Bill E., Schwenn J. D., Seidler A. A dimer of the FeS cluster biosynthesis protein IscA from cyanobacteria binds a [2Fe2S] cluster between two protomers and transfers it to [2Fe2S] and [4Fe4S] apo proteins. Eur. J. Biochem. 2003;270:1662–1671. doi: 10.1046/j.1432-1033.2003.03522.x. [DOI] [PubMed] [Google Scholar]
- 31.Morimoto K., Sato S., Tabata S., Nakai M. A HEAT-repeats containing protein, IaiH, stabilizes the iron-sulfur cluster bound to the cyanobacterial IscA homologue, IscA2. J. Biochem. (Tokyo) 2003;134:211–217. doi: 10.1093/jb/mvg131. [DOI] [PubMed] [Google Scholar]
- 32.Morimoto K., Nishio K., Nakai M. Identification of a novel prokaryotic HEAT-repeats-containing protein which interacts with a cyanobacterial IscA homolog. FEBS Lett. 2002;519:123–127. doi: 10.1016/s0014-5793(02)02736-9. [DOI] [PubMed] [Google Scholar]
- 33.Ollagnier-De-Choudens S., Sanakis Y., Fontecave M. SufA/IscA: reactivity studies of a class of scaffold proteins involved in [Fe-S] cluster assembly. J. Biol. Inorg. Chem. 2004;9:828–838. doi: 10.1007/s00775-004-0581-9. [DOI] [PubMed] [Google Scholar]
- 34.Wu G., Mansy S. S., Hemann C., Hille R., Surerus K. K., Cowan J. A. Iron-sulfur cluster biosynthesis: characterization of Schizosaccharomyces pombe Isa1. J. Biol. Inorg. Chem. 2002;7:526–532. doi: 10.1007/s00775-001-0330-2. [DOI] [PubMed] [Google Scholar]
- 35.Cozar-Castellano I., Del Valle Machargo M., Trujillo E., Arteaga M. F., Gonzalez T., Martin-Vasallo P., Avila J. hIscA: a protein implicated in the biogenesis of iron-sulfur clusters. Biochim. Biophys. Acta. 2004;1700:179–188. doi: 10.1016/j.bbapap.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Wu S. P., Cowan J. A. Iron-sulfur cluster biosynthesis. A comparative kinetic analysis of native and Cys-substituted ISA-mediated [2Fe-2S]2+ cluster transfer to an apoferredoxin target. Biochemistry. 2003;42:5784–5791. doi: 10.1021/bi026939+. [DOI] [PubMed] [Google Scholar]
- 37.Bilder P. W., Ding H., Newcomer M. E. Crystal structure of the ancient, Fe-S scaffold IscA reveals a novel protein fold. Biochemistry. 2004;43:133–139. doi: 10.1021/bi035440s. [DOI] [PubMed] [Google Scholar]
- 38.Cupp-Vickery J. R., Silberg J. J., Ta D. T., Vickery L. E. Crystal structure of IscA, an iron-sulfur cluster assembly protein from Escherichia coli. J. Mol. Biol. 2004;338:127–137. doi: 10.1016/j.jmb.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 39.Bertini I., Cowan J. A., Del Bianco C., Luchinat C., Mansy S. S. Thermotoga maritima IscU. Structural characterization and dynamics of a new class of metallochaperone. J. Mol. Biol. 2003;331:907–924. doi: 10.1016/s0022-2836(03)00768-x. [DOI] [PubMed] [Google Scholar]
- 40.Adinolfi S., Rizzo F., Masino L., Nair M., Martin S. R., Pastore A., Temussi P. A. Bacterial IscU is a well folded and functional single domain protein. Eur. J. Biochem. 2004;271:2093–2100. doi: 10.1111/j.1432-1033.2004.04112.x. [DOI] [PubMed] [Google Scholar]
- 41.Johnson D. C., Dos Santos P. C., Dean D. R. NifU and NifS are required for the maturation of nitrogenase and cannot replace the function of isc-gene products in Azotobacter vinelandii. Biochem. Soc. Trans. 2005;33:90–93. doi: 10.1042/BST0330090. [DOI] [PubMed] [Google Scholar]
- 42.Nuth M., Yoon T., Cowan J. A. Iron-sulfur cluster biosynthesis: characterization of iron nucleation sites for assembly of the [2Fe-2S](2+) cluster core in IscU proteins. J. Am. Chem. Soc. 2002;124:8774–8775. doi: 10.1021/ja0264596. [DOI] [PubMed] [Google Scholar]
- 43.Yang W., Rogers P. A., Ding H. Repair of nitric oxide modified ferredoxin [2Fe-2S] cluster by cysteine desulfurase (IscS) J. Biol. Chem. 2002;277:12868–12873. doi: 10.1074/jbc.M109485200. [DOI] [PubMed] [Google Scholar]
- 44.Yu L., Kennedy M., Czaja C., Tavares P., Moura J. J., Moura I., Rusnak F. Conversion of desulforedoxin into a rubredoxin center. Biochem. Biophys. Res. Commun. 1997;231:679–682. doi: 10.1006/bbrc.1997.6171. [DOI] [PubMed] [Google Scholar]
- 45.Cowart R. E., Singleton F. L., Hind J. S. A comparison of bathophenanthrolinedisulfonic acid and ferrozine as chelators of iron(II) in reduction reactions. Anal. Biochem. 1993;211:151–155. doi: 10.1006/abio.1993.1246. [DOI] [PubMed] [Google Scholar]
- 46.Srinivasan C., Liba A., Imlay J. A., Valentine J. S., Gralla E. B. Yeast lacking superoxide dismutase(s) show elevated levels of ‘free iron’ as measured by whole cell electron paramagnetic resonance. J. Biol. Chem. 2000;275:29187–29192. doi: 10.1074/jbc.M004239200. [DOI] [PubMed] [Google Scholar]
- 47.Jensen L. T., Culotta V. C. Role of Saccharomyces cerevisiae ISA1 and ISA2 in iron homeostasis. Mol. Cell. Biol. 2000;20:3918–3927. doi: 10.1128/mcb.20.11.3918-3927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaut A., Lange H., Diekert K., Kispal G., Lill R. Isa1p is a component of the mitochondrial machinery for maturation of cellular iron-sulfur proteins and requires conserved cysteine residues for function. J. Biol. Chem. 2000;275:15955–15961. doi: 10.1074/jbc.M909502199. [DOI] [PubMed] [Google Scholar]
- 49.Pelzer W., Muhlenhoff U., Diekert K., Siegmund K., Kispal G., Lill R. Mitochondrial Isa2p plays a crucial role in the maturation of cellular iron-sulfur proteins. FEBS Lett. 2000;476:134–139. doi: 10.1016/s0014-5793(00)01711-7. [DOI] [PubMed] [Google Scholar]
- 50.Jones D. P., Carlson J. L., Mody V. C., Cai J., Lynn M. J., Sternberg P. Redox state of glutathione in human plasma. Free Radical Biol. Med. 2000;28:625–635. doi: 10.1016/s0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- 51.Taylor J. E., Yan J. F., Wang J. L. The iron(3)-catalyzed oxidation of cysteine by molecular oxygen in the aqueous phase. An example of a two-thirds-order reaction. J. Am. Chem. Soc. 1966;88:1663–1667. doi: 10.1021/ja00960a016. [DOI] [PubMed] [Google Scholar]
- 52.McAuliffe C. A., Murray S. G. Metal complexes of sulphur-containing amino acids. Inorg. Chim. Acta Rev. 1972;6:103–121. [Google Scholar]
- 53.Greenberg J. T., Demple B. Glutathione in Escherichia coli is dispensable for resistance to H2O2 and gamma radiation. J. Bacteriol. 1986;168:1026–1029. doi: 10.1128/jb.168.2.1026-1029.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park S., Imlay J. A. High levels of intracellular cysteine promote oxidative DNA damage by driving the Fenton reaction. J. Bacteriol. 2003;185:1942–1950. doi: 10.1128/JB.185.6.1942-1950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]