Abstract
Annexin A13 is considered the original progenitor of the 11 other members of vertebrate annexins, a superfamily of calcium/phospholipid-binding proteins. It is highly tissue-specific, being expressed only in intestinal and kidney epithelial cells. Alternative splicing generates two isoforms, both of which bind to rafts. In view of the lack of structural information supporting the physiological role of this annexin subfamily, we have cloned, expressed and purified human annexin A13b to investigate its structural and functional properties. The N-terminus of annexin A13b: (i) destabilizes the conserved protein core, as deduced from the low melting temperature in the absence (44 °C) or presence of calcium (55 °C), and (ii) impairs calcium-dependent binding to acidic phospholipids, requiring calcium concentrations >400 μM. Truncation of the N-terminus restores thermal stability and decreases the calcium requirement for phospholipid binding, confirming its essential role in the structure–function relationship of this annexin. Non-myristoylated annexin A13b only binds to acidic phospholipids at high calcium concentrations. We show for the first time that myristoylation of annexin A13b enables the direct binding to phosphatidylcholine, raft-like liposomes and acidic phospholipids in a calcium-independent manner. The conformational switch induced by calcium binding, from a ‘closed’ to an ‘open’ conformation with exposure of Trp227, can be mimicked by a decrease in pH, a process that may be relevant for membrane interactions. Our studies confirm that the common structural and functional characteristics that are dependent on the protein core of vertebrate annexins are likely to be common conserved features, whereas their variable N-termini confer distinct functional properties on annexins, as we report for myristoylation of annexin A13b.
Keywords: annexin A13, calcium binding, CD spectroscopy, fluorescence spectroscopy, phospholipid binding, thermal stability
Abbreviations: Anx, annexin; CCA, convex constraint algorithm; IPTG, isopropyl β-D-thiogalactoside; LB, Luria–Bertani; MALDI–TOF MS, matrix-assisted laser-desorption ionization–time-of-flight mass spectrometry; PC, phosphatidylcholine; PG, phosphatidylglycerol; PS, phosphatidylserine; Tm, melting temperature; λex, excitation wavelength
INTRODUCTION
The annexin superfamily comprises a group of calcium- and phospholipid-binding proteins characterized by a common structural arrangement, the annexin core. This core is defined by a tetrad structure of homologous internal repeats that mediates the reversible calcium-dependent binding to biological membranes [1–3]. It is composed of four repeats [eight in AnxA6 (annexin A6)] universally conserved throughout annexin evolution [4,5]. Each of them is formed by a four anti-parallel α-helix (A, B, D and E) bundle capped by helix C, as assessed from the crystal structure of several family members. The four domains arrange around a central hydrophilic pore giving rise to a slightly bent disc with calcium- and membrane-binding sites located to the convex side [6]. Calcium binds mainly to the well characterized type II calcium-binding sites [1–3]. However, additional calcium- and phospholipid-binding sites have been described, highlighting the complexity of annexin interactions with membranes [7,8]. In contrast with the conserved core, the N-terminus is variable in both length and amino acid sequence, and is considered to be a major regulatory region of annexins [9,10].
AnxA13 is a key member of vertebrate annexins. The relationship of its gene organization with that of protists and plants indicates that it is the most ancient member of vertebrate annexins [11]. The consequent evolutionary steps originate the other members of the family, beginning with the duplication to AnxA7 and AnxA11 [12–14]. A short ‘a’ isoform was first identified as a gut-specific annexin by Wice and Gordon [15]; alternative splicing at the N-terminus originates the ‘b’ isoform, with an insertion of 41 residues [16]. Another peculiar feature of this subfamily is the presence of an N-terminal sequence that leads to the N-myristoylation of the terminal glycine residue, a post-translational modification that is unique among vertebrate annexins.
Functionality of annexins has been widely discussed. Several in vitro roles have been assigned to these proteins, such as anti-coagulatory and anti-inflammatory activities, involvement in calcium signal transduction, membrane fusion, and endo- and exocytosis, as well as calcium channel regulation, although their in vivo functions are just beginning to be understood [1–3]. This functional diversity can be attributed to specific gene regulation, expression patterns or subcellular localization. It can be also explained in terms of differences in the N-terminal region which mediates the interaction with other proteins, undergoes several posttranslational modifications and influences the overall structure of the protein [9,10]. Most of these observations are based on the calcium-dependent binding to membranes; however, a lot of attention is being focused lately on the possible calcium-independent interaction of annexins with phospholipids and on the influence of mildly acidic pH on the structure and possible insertion of annexins into the bilayer.
The specific role of AnxA13 is of particular interest, as its expression is limited to epithelial cells and its localization is also specific. The AnxA13a isoform localizes to both apical and basolateral membranes, but AnxA13b is enriched in the apical region in the lower three-quarters of the microvilli of polarized epithelial cells [17,18]. Moreover, both isoforms associate with detergent-resistant lipid microdomains, the so-called rafts. In good agreement with these results, AnxA13a has been implicated in both apical and basolateral transport, while AnxA13b only takes part in apical transport [19,20]. N-myristoylation appears to be essential for these roles, whereas calcium seems to be required only for the association of AnxA13a to basolateral transport vesicles. The co-localization of AnxA13b with KIFC3, a minus-end-directed motor, supports further the participation of this protein in the formation and docking of vesicles from the trans-Golgi network to the apical membrane [19,21]. Furthermore, AnxA13b interacts with Nedd4, directing it to the apical membrane and to raft microdomains [22].
In the present study, we performed a structural characterization of AnxA13b considering the effect of calcium binding, the main modulator of its activity. Taking into account that mildly acidic pH values trigger important conformational changes in Anx A5 and Hydra AnxB12, we have also analysed the effect of increasing proton concentration on AnxA13b structure. Additionally, we have been able to obtain the protein core of AnxA13 as well as the myristoylated AnxA13b in order to characterize the binding properties of these proteins with different phospholipid vesicles. As we have mentioned before, AnxA13 is the key between the ‘A’ family and the rest of annexins; it is therefore worth studying the structural and functional characteristics of this annexin in order to determine whether some of the common properties of these proteins result from a convergent evolution or rather they constitute a shared and conserved property maintained throughout evolution.
EXPERIMENTAL
Construction of AnxA13b expression vector
The sequence for human AnxA13b cDNA has been completed and annotated previously (IMAGE: 3349423; GenBank® accession number AJ306450). This sequence, encoding the full-length cDNA, was used as template to amplify and isolate the coding portion by PCR. Oligonucleotide primers 22 bp long were synthesized according to the published sequence for both ends of the coding cDNA. The oligonucleotide sequences included created NcoI and HindIII restriction sites to facilitate the insertion of coding cDNA into the expression plasmid pTrc99A (Amersham Biosciences, Bucks., U.K.). The identity of the final construct pTrcA13Hb was verified by DNA restriction analysis and sequencing.
Protein expression and purification
JA221 Escherichia coli cells were transformed with the human AnxA13b expression construct pTrcA13Hb and grown in LB (Luria–Bertani) broth containing 100 μg/ml ampicillin at 37 °C until a D600 of 0.5 was reached. Optimal expression induction was obtained by addition of 1 mM IPTG (isopropyl β-D-thiogalactoside) for 3 h at 37 °C. Purification of the recombinant protein was achieved by taking advantage of the property of being able to reversibly interact with PS (phosphatidylserine) vesicles and following, with slight modifications, the method described for AnxA5 [23]. Briefly, after centrifugation at 6000 g for 15 min, collected cells were resuspended in 50 mM Tris/HCl, pH 7.4, containing 0.1 M NaCl, 2.5 mM EGTA and 2 mM PMSF and ruptured by sonication cycles at 4 °C. The homogenate was stirred at 4 °C for 90 min and centrifuged at 27000 g for 90 min at 4 °C. The supernatant was mixed with PS-enriched vesicles (bovine brain extract with approx. 80% PS; 1 mg/ml final concentration; Sigma, Alcobendas, Spain) and 2 mM CaCl2, and stirred for 30 min at 4 °C. The mixture was centrifuged at 27000 g for 45 min at 4 °C, and the pellet washed twice with 50 mM Tris/HCl, pH 7.4, containing 0.1 M NaCl and 2 mM CaCl2. The recombinant protein was then extracted by resuspension of the vesicles in the presence of 50 mM Tris/HCl, pH 7.4, containing 5 mM EGTA and 0.25 M NaCl, and further centrifugation at 27000 g for 45 min at 4 °C. Finally, the protein was purified, after dialysis against 50 mM Tris/HCl, pH 7.4, and 1 mM EGTA, by DEAE-cellulose chromatography; AnxA13b was eluted using buffer containing 0.25 M NaCl and 1 mM EGTA.
Limited proteolysis of AnxA13b was performed in 50 mM Tris/HCl, pH 8.0, and 0.1 M NaCl with sequence-grade tosylphenylalanylchloromethane-treated trypsin (Roche Diagnostics, Mannheim, Germany) at an enzyme/substrate ratio of 1:1000 (w/w) at 25 °C for 2 h. The reaction was stopped by the addition of 1 mM PMSF, and the truncated protein (AnxA13core) was purified by size-exclusion chromatography on a Sephadex G-75 column (Amersham Biosciences).
The identity of the recombinant (AnxA13b) and N-terminally truncated (AnxA13core) proteins was confirmed by amino acid analysis (Beckman 6300 amino acid analyser) as described previously [23], as well as N-terminal sequencing and MALDI–TOF MS (matrix-assisted laser-desorption ionization–time-of-flight mass spectrometry). The molar absorption coefficient at 280 nm was determined from the UV/visible spectrum after subtraction of apparent absorption due to light scattering and determination of the protein concentration by amino acid analyses from aliquots directly taken from the cuvette (ϵ0.1%=0.81 and 0.75 ml·mg−1·cm−1 for AnxA13b and AnxA13core respectively).
Myristoylation of recombinant AnxA13b was performed in JA221 E. coli cells harbouring plasmid constructs encoding N-myristoyltransferase from Saccharomyces cerevisiae (pBB131 containing the gene for kanamycin resistance and also inducible by IPTG [24]; kindly provided by Dr J. I. Gordon, Department of Molecular Biology and Pharmacology, Washington University School of Medicine, St. Louis, MO, U.S.A.) and human AnxA13b. Cultures of the double transformants were shaken at 37 °C to a D600 of 0.5 in LB broth containing 100 μg/ml ampicillin and 100 μg/ml kanamycin sulphate. Simultaneous expression of both recombinant proteins was triggered by the addition of 1 mM IPTG for 4 h at 37 °C. Protein radiolabelling was assessed by simultaneous addition of IPTG and [9,10(n)-3H]myristic acid (Amersham Biosciences) (55.0 Ci/mmol, 10 μCi/ml of culture). Non-myristoylated AnxA13b was mainly solubilized as described above, whereas the myristoylated protein (AnxA13bmyr) remained in the pellet from the bacterial homogenization. Solubilization was achieved by addition of 2% (w/v) octylglucoside; partial purification was performed by DEAE-cellulose chromatography after dialysis against 50 mM Tris/HCl, pH 7.4, containing 0.5% (w/v) octylglucoside, and elution with increasing NaCl concentration. Finally, octylglucoside was removed by exhaustive dialysis against 50 mM Tris/HCl, pH 7.4, and 0.1 M NaCl, as described previously [25].
CD spectroscopy
The far-UV CD spectra were monitored between 195 and 260 nm at 20 °C in a Jasco J-715 spectropolarimeter equipped with a Neslab RTE-111 thermostat using 0.01 or 0.05 cm path-length thermostatically controlled cuvettes. All spectra were averaged over at least six scans and were corrected by subtracting buffer contribution from parallel spectra in the absence of protein; units are expressed as mean residue weighed molar ellipticities ([θ]MRW). Prediction of the secondary structure from the far-UV CD spectra was performed using the CCA (convex constraint algorithm) as described by Perczel et al. [26]. Melting curves were obtained after dialysis of the samples against 20 mM Hepes, pH 7.4, containing 0.1 M NaCl to avoid pH changes during heating. Changes in ellipticity at 208 nm were monitored between 20 and 85 °C at 60 °C/h. Ellipticity data were converted into the apparent fraction of unfolded protein (Fapp) and plotted against temperature according to Fapp=(θobs−θN)/(θU−θN), where θobs is the observed ellipticity value at a given temperature, and θN and θU are the values for native and unfolded forms respectively, determined at each temperature by linear extrapolation of the native and unfolded baselines.
The effect of calcium on the far-UV CD spectrum and melting temperature (Tm; considered as the midpoint of the unfolding transition calculated from the plot of Fapp against temperature) was analysed employing protein samples with increasing CaCl2 concentrations. Samples with equivalent maximal ionic strength, obtained by the addition of NaCl or MgCl2 instead of CaCl2, were used as controls. Control samples without calcium always contained 1 mM EGTA. The effect of acidic pH was studied by dilution of a concentrated protein stock with 20 mM sodium citrate buffer containing 0.1 M NaCl at different pH values [27], and the exact pH values after dilution were determined using a microelectrode. Spectra in the presence of pure brain-extract PS liposomes (50 nm in diameter, obtained by extrusion as described below; 800:1 PS/protein molar ratio) were recorded in the absence or presence of 750 μM CaCl2 or MgCl2 using a 0.01 cm path-length cuvette to minimize artifacts arising from light scattering.
Fluorescence emission spectroscopy
Fluorescence emission spectra were recorded in an SLM Aminco 8000C spectrofluorimeter at 20 °C. Global and tryptophan emissions were measured separately with excitation wavelengths of 275 and 295 nm respectively, using a 0.4 cm excitation path-length and 1.0 cm emission path-length cuvette. Spectra were monitored between 265 and 420 nm for global emission or 305 and 420 nm for tryptophan emission. Rayleigh scattering at 90° was obtained from the maximum at 275 nm of spectra recorded at this excitation wavelength. The emission spectrum from tyrosine residues was determined after subtracting the contribution of the tryptophan residue to the global emission spectra [λex (excitation wavelength)=275 nm]; this contribution was obtained by correction of the tryptophan spectrum (λex=295 nm) using a factor determined from the ratio between both spectra (Fex=275/Fex=295) in the range 370–400 nm.
The effect of calcium was titrated by sequential addition of CaCl2 in the absence or presence of 50 nm unilamellar PS vesicles (800:1 PS/AnxA13b molar ratio). The effect of acidic pH was studied as described for CD spectroscopy. Light scattering was minimized by crossed Glan–Thompson polarizers only in spectra obtained in the presence of liposomes.
Acrylamide quenching of tryptophan fluorescence was analysed by recording emission spectra (λex=295 nm) at increasing acrylamide concentrations, and taking into account the effect of dilution as described previously [23,28]. Care was taken to avoid the inner-filter effect and that solutions always presented UV absorption below 0.04 at 295 nm. The quenching constant (KSV) was calculated from the Stern–Volmer plot of F0/F at the emission maximum against acrylamide concentration, according to the equation F0/F=1+KSV[Q], where F0 is the fluorescence intensity at zero quencher concentration, and F is the intensity at a given quencher concentration ([Q]).
Binding to phospholipids and vesicle aggregation assays
Unilamellar vesicles were prepared using natural lipids (purity>99%): PS (bovine brain) (Avanti Polar Lipids, Alabaster, AL, U.S.A.), PC (phosphatidylcholine) (egg yolk), PG (phosphatidylglycerol) (derived from egg-yolk PC), and a raft-like mixture (PC/sphingomyelin/cholesterol, 1:1:1) (Sigma). Liposomes were obtained by hydration of a thin dried-lipid film in buffer (50 mM Tris/HCl or 20 mM Hepes, pH 7.4) containing 0.1 M NaCl, followed by extrusion through polycarbonate filters of either 100 or 400 nm pore diameter (Lipex Biomembranes, Vancouver, Canada). Small unilamellar vesicles (50 nm) were prepared from freshly obtained 400 nm vesicles by further extrusion through polycarbonate filters of the corresponding pore diameter.
The interaction of AnxA13b or AnxA13core with 400 nm vesicles was carried out at a constant lipid/protein molar ratio of 800:1 with variable calcium concentrations in 50 mM Tris/HCl, pH 7.4, containing 0.1 M NaCl at 20 °C for 15 min. The final mixture (300 μl) was ultracentrifuged at 110000 rev./min at 4 °C for 1 h (Airfuge Beckman; A110 rotor). After separation of supernatant and pellet, proteins were detected by Coomassie Blue staining after SDS/PAGE. Phospholipid-free controls showed negligible sedimentation of annexin under these experimental conditions. Gels were scanned, and a densitometric analysis was performed, obtaining volumograms on a photodocumentation system from UVItec (Cambridge, U.K.) and using the UVIBand V.97 software. [3H]Myristoylated AnxA13b binding was analysed as described for the non-myristoylated form; quantification was carried out additionally by measuring radioactivity in supernatants and pellets.
Vesicle-aggregation studies were performed by recording absorption at 360 nm immediately after the addition of calcium to a mixture of unilamellar vesicles (100 nm; 0.1 mg/ml) and AnxA13b at 25 °C. Calcium was added from a CaCl2 stock solution, and absorption was registered in a thermostatically controlled cuvette for at least 10 min.
Structural model
We have generated a three-dimensional model using the Swiss-Model Server version 3.5 (http://swissmodel.expasy.org/) and public X-ray crystallographic data for full-length pig AnxA1, human AnxA2 and AnxA3, human and rat AnxA5, and Hydra AnxB12 (Protein Data Bank entries 1MCX, 1XJL, 1AXN, 1ANX, 1A8A and 1AEI respectively), all of them in the ‘high-calcium’ form to thread the human AnxA13b amino acid sequence into a three-dimensional model for the homologous tetrad core region. Additionally, we have also obtained a structural model for AnxA13b domain III in the absence of calcium using the crystallographic data from pig AnxA1, human AnxA2, bovine AnxA4, and human AnxA5 and AnxA8 (Protein Data Bank entries 1HM6, 1W7B, 1ANN, 1AVH and 1W3W respectively).
Other procedures
SDS/PAGE was performed according to Laemmli [29] using 10% polyacrylamide gels. Western blotting was performed as described previously [28] using rabbit polyclonal anti-(human AnxA13b) antibodies and a secondary anti-rabbit IgG conjugated with horseradish peroxidase (Bio-Rad, Madrid, Spain). Polyclonal antibodies against recombinant human AnxA13b were raised in rabbits following standard methods. Radioactivity was measured in a Beckman LS 3801 scintillation counter. Autoradiography was performed after soaking the SDS/PAGE gels in the fluorographic reagent Amplify (Amersham Biosciences), drying the gels and exposure to Hyperfilm-MP films (Amersham Biosciences). Protein concentration in pure preparations was determined from the UV/visible spectra, except for CD spectroscopy, where quantitative amino acid analysis was used. Regression fitting of the experimental data to different equations was performed using SigmaPlot software version 9.01 obtained from Systat Software (Erkrath, Germany).
RESULTS
Purification of recombinant AnxA13b
The expression of recombinant AnxA13b was studied in JA221 E. coli cells transformed with the expression construct pTrcA13Hb. Protein production was high at short induction times (1 mM IPTG), and recombinant AnxA13b was detectable by SDS/PAGE and Coomassie Blue staining (Figure 1A, lane H). Longer times did not yield greater amounts of protein, but were accompanied by a significant increase in protein degradation, as detected by electrophoretic analysis and Western blotting (results not shown). Consequently, 3 h of induction at 37 °C was chosen as the optimal conditions for protein expression.
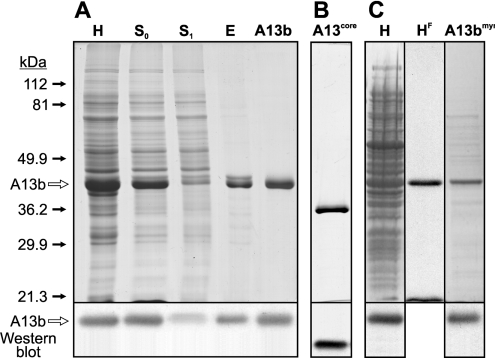
Figure 1. SDS/PAGE analysis of recombinant non-myristoylated and myristoylated AnxA13b expression and purification.
(A) Expression of AnxA13b was analysed by SDS/PAGE, followed by Coomassie Blue staining and Western blotting. Lane H, bacterial homogenate after protein expression induction; lane S0, supernatant after centrifugation of the homogenized material in the presence of 2.5 mM EGTA; lane S1, supernatant after addition of PS-enriched liposomes in the presence of 2 mM CaCl2; lane E, supernatant obtained after extraction with 5 mM EGTA of the complex between AnxA13b and PS-enriched liposomes; lane A13b, pool of fractions obtained after DEAE-cellulose chromatography. (B) Lane A13core, protein core of AnxA13b. (C) Expression of myristoylated AnxA13b was analysed by SDS/PAGE followed by Coomassie Blue staining, fluorography and Western blotting. Lane H, bacterial homogenate after protein induction; lane HF, fluorography of sample from lane H; lane A13bmyr, pool of fractions containing purified myristoylated AnxA13b after DEAE-cellulose chromatography and removal of octylglucoside. Molecular masses in kDa and the position of A13b are indicated.
The electrophoretic and Western blot analyses of the main steps of the purification process are shown in Figure 1(A). Homogenization of the bacterial pellet in the presence of 2.5 mM EGTA (Figure 1A, lane H) allowed the solubilization of the recombinant protein with a high yield (Figure 1A, lane S0). Sequential addition of multilamellar PS-enriched vesicles and CaCl2 (2 mM final concentration) followed by centrifugation sedimented the recombinant protein, whereas the main part of bacterial proteins remained in the supernatant (Figure 1A, lane S1). AnxA13b was extracted from the vesicle pellet with buffer containing 5 mM EGTA and 0.25 M NaCl (Figure 1A, lane E); the higher NaCl concentration required to extract the protein from the vesicles emphasizes the importance of ionic interactions in membrane binding [8,30]. The remaining contaminant proteins and phospholipidic vesicles were removed with the DEAE-cellulose chromatographic step (Figure 1A, lane A13b). Controlled trypsin digestion of purified recombinant AnxA13b followed by size-exclusion chromatography yielded AnxA13core, a truncated form without the first 48 amino acid residues (cut after Lys48; Figure 1B, lane A13core). The identity and purity of the protein preparations were confirmed by Western blotting, N-terminal sequencing (12 residues) and MALDI–TOF MS.
Taking into account that AnxA13b is myristoylated in vivo, we considered the production of myristoylated recombinant protein in order to compare its ability to bind to phospholipids with that of the non-myristoylated protein. Figure 1(C) (lane H) shows that the co-transformation of the E. coli JA221 strain with pTrcA13Hb and pBB131 yielded recombinant protein that incorporated [3H]myristic acid. Moreover, the only myristoylated protein detected by fluorography was the recombinant annexin (Figure 1C, lane HF). The myristoylated protein was quite insoluble under the experimental conditions described for the solubilization of non-myristoylated AnxA13b, allowing, in this way, a good separation of both forms of the recombinant protein. The low solubility of the myristoylated protein is probably due to a calcium-independent binding to bacterial membranes. Solubilization using 2% (w/v) octylglucoside, followed by DEAE-cellulose chromatography and dialysis, yielded final preparations of soluble radiolabelled AnxA13bmyr that were approx. 80% pure (Figure 1C, lane A13bmyr), and were used for phospholipid-binding experiments.
CD analysis and thermal stability
CD spectra of AnxA13b in the far-UV region in the absence of calcium, at 20 and 85 °C, are shown in Figure 2(A) (continuous lines). The shape of the spectrum at 20 °C reflects a high α-helical percentage in the secondary structure of the protein, with two minima at 208 and 221 nm. Accordingly, the CCA prediction indicates that the main contributions to the secondary structure are α-helix (70–75%) and random coil (15–18%), in good agreement with the secondary structure of the protein core of other annexins whose three-dimensional structures are already known. Annexins with a short N-terminus, such as AnxA5 (15 residues), present CD and IR spectra with a prediction of over 80% α-helical structure [31], whereas AnxA11, with a 200-residue extension in random coil, presents only 29% α-helix [28]. Taking these data into account, and considering that predictions based on the sequence of the N-terminus of AnxA13b (51 residues) suggest a random coil structure (secondary-structure prediction tools at http://www.expasy.org/tools/#secondary), it can be suggested that the α-helical contribution to the CD spectra corresponds only to the protein core. In fact, truncation of the N-terminus (A13core) alters the far-UV CD spectrum, with less negative ellipticity values, but showing a decrease in random coil structures and a parallel increase in α-helical content according to the CCA prediction.
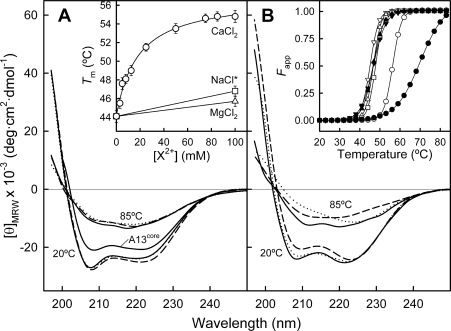
Figure 2. Far-UV CD spectra of AnxA13b in the absence and presence of PS unilamellar vesicles.
(A) Far-UV CD spectra of AnxA13b (0.3 mg/ml) without calcium (continuous line) and in the presence of 100 mM CaCl2 (broken line) or MgCl2 (dotted line) at 20 and 85 °C are shown. Spectra were registered in 20 mM Hepes, pH 7.4, containing 0.1 M NaCl. Inset: dependence of the Tm on CaCl2 concentration ranging from 0 (1 mM EGTA) to 100 mM (○), 100 mM MgCl2 (△) or NaCl with ionic strength equivalent to buffer containing 100 mM CaCl2 (NaCl*; □). Melting points represent the means±S.D. for at least two independent determinations. (B) Far-UV CD spectra of AnxA13b as described above, but in the presence of 50 nm PS unilamellar liposomes (800:1 lipid/protein molar ratio) without (continuous line) or with 750 μM CaCl2 (broken line) or MgCl2 (dotted line). Inset: apparent fraction of unfolded protein (Fapp) against temperature (open symbols, in the absence of PS; closed symbols, in the presence of PS vesicles; down triangles, EGTA; circles, CaCl2; up triangles, MgCl2; squares, NaCl).
The addition of CaCl2 up to 100 mM induces very slight changes in the CD spectrum of AnxA13b (Figure 2A, broken lines), smaller than those detected in AnxA5 and AnxA11 [28,31], but pointing also to a small increase in α-helix content (<5%) and helix length (higher [θ]220/[θ]208 ratio).
Since the main contribution to the secondary structure is α-helix, thermal denaturation was followed by measuring the molar ellipticity at 208 nm as a function of temperature. The AnxA13b melting curve displays a co-operative transition with a Tm of approx. 44 °C which is approx. 6 °C lower than the value obtained for AnxA13core (Figure 2B, inset). Addition of calcium induces a concentration-dependent increase in the thermal stability of AnxA13b that follows a hyperbolic pattern with a midpoint at 12.8 mM and a maximum increase of approx. 10–11 °C at concentrations higher than 50 mM (Figure 2A, inset). This effect is calcium-specific and cannot be reproduced by magnesium or by an equivalent increase in ionic strength achieved by addition of NaCl. The study of calcium binding in the absence of phospholipids requires the use of millimolar concentrations of CaCl2, as it is well known that the affinity of annexins for this cation is around three orders of magnitude lower under these conditions [31,32].
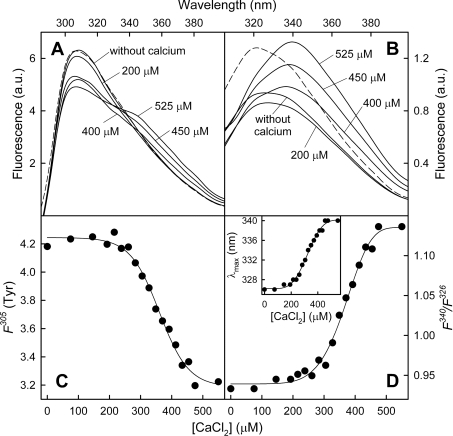
Fluorescence emission analysis
Fluorescence emission spectra of AnxA13b were registered with excitation wavelengths of 275 nm (global emission) (Figure 3A) and 295 nm (Trp227 emission) (Figure 3B) in the presence of different calcium concentrations. In the absence of calcium, the maximum is around 307 nm and 321 nm for global and tryptophan emission, respectively. Addition of calcium up to 75 mM induces a significant shift in the Trp227 maximum towards 344 nm (Figure 3D, inset), whereas only a small shift (from 307 to 303 nm) is detected in the global maximum. When the contribution of tyrosine residues to the global spectra is analysed (Figure 3C, F305, ●), a very slight change in fluorescence intensity at 305 nm is observed, being almost identical to that obtained with MgCl2 (Figure 3C, F305, ○). On the other hand, Trp227 emission follows a hyperbolic dependence on calcium concentration in both the position of the maximum and in the ratio between intensities at 344 and 321 nm (Figure 3D). This effect is calcium-specific and is not reproducible by magnesium, with a midpoint effect at 2.7 mM CaCl2 for the F344/F321 ratio and 1 mM for the shift in the maximum position. Additionally, we have recorded Rayleigh scattering (Figure 3C, RS275) as a function of calcium or magnesium concentration, and no significant variation has been found, thus indicating that calcium effects are not due to induction of protein aggregation.
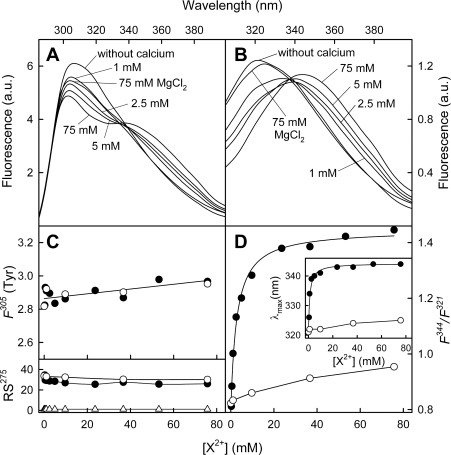
Figure 3. Fluorescence emission spectra of AnxA13b after calcium binding.
Emission spectra at excitation wavelengths of 275 (A) and 295 (B) nm in the absence of calcium or at increasing CaCl2 concentration were registered at 20 °C; protein concentration was 0.2 mg/ml. Only spectra at representative calcium concentrations are shown. Fluorescence is expressed in arbitrary units (a.u.). Variations in the fluorescence intensity of tyrosine residues at 305 nm [F305 (Tyr); determined as described in the Experimental section] and in the F344/F321 fluorescence intensity ratio of Trp227 (λex=295 nm) with Ca2+ (●) or Mg2+ (○) concentration are shown in (C) and (D) respectively. The lower panel in (C) represents Rayleigh scattering at 275 nm (RS275) of AnxA13b or buffer (△) at different Ca2+ or Mg2+ concentrations. The inset in (D) shows the variation in the tryptophan fluorescence maximum position with calcium concentration.
The exposure of Trp227 to the solvent was studied in the absence and in the presence of 75 mM CaCl2 through analysis of the sensibility of fluorescence emission (λex=295 nm) to acrylamide quenching up to 170 mM, which does not involve inner-filter effects. Accessibility to acrylamide was confirmed by the decrease in intensity observed in the spectra in both cases, without modification in the position of the maximum (results not shown). The fluorescence emission data at the corresponding maximum (321 or 344 nm) were adjusted according to the Stern–Volmer equation as a function of acrylamide concentration, yielding quenching constants (KSV) of 2.99 and 14.58 M−1 in the absence or presence of 75 mM CaCl2 respectively.
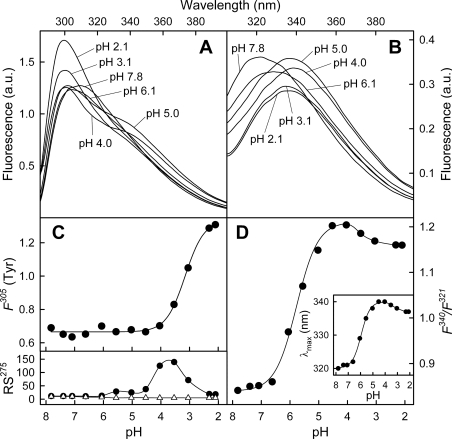
Effect of acidic pH on AnxA13b conformation
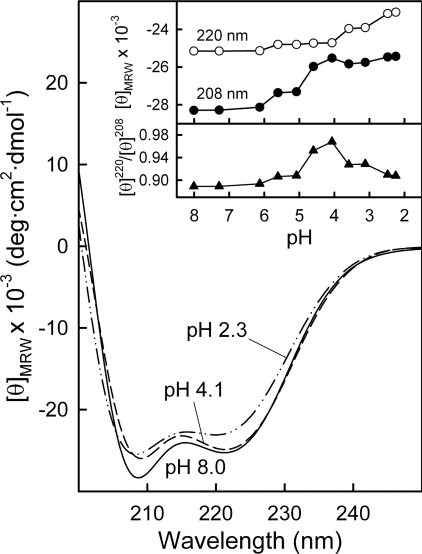
CD spectra of AnxA13b in the far-UV region were registered at decreasing pH, beginning at 8.0 (Figure 4, continuous line), at approx. 0.1 mg/ml to minimize aggregation of the protein at pH values close to the pI (5.8) or lower. No significant alterations in the secondary structure were detected down to pH 5.0. However, at more acidic pH, changes were observed (Figure 4, upper inset) first in the minimum at 208 nm (down to pH 4.1; Figure 4, broken line) followed by changes at 220 nm (down to pH 2.3; Figure 4, broken/dotted line). The overall changes are reflected in a moderate increase in the [θ]220/[θ]208 ratio that reaches a maximum at pH 4.1. The decrease thereafter (Figure 4, lower inset) is accompanied by a loss in negative molar ellipticity consistent with an increase in random coil and a parallel decrease in α-helix percentage of approx. 15–20%.
Figure 4. Far-UV CD spectra of AnxA13b as a function of pH.
Spectra were recorded at 20 °C using AnxA13b at 0.1 mg/ml in sodium citrate buffer solutions at decreasing pH values, as described in the Experimental section. Only representative spectra at pH 8.0, 4.1 and 2.3 are shown. The inset shows the plot of ellipticity values at 220 and 208 nm against pH (upper panel) and the ratio between these values (lower panel).
Fluorescence emission spectra at excitation wavelengths of 275 and 295 nm were recorded at different pH values (Figures 5A and 5B). The contribution of the 12 tyrosine residues to the global emission spectra was analysed after subtracting that of Trp227. Figure 5(C) (F305) shows the effect of decreasing pH in the fluorescence emission of tyrosine residues at 305 nm. No variations in the intensity were observed from pH 7.8 to approx. pH 4.0, whereas a sharp increase was detected at lower pH values. Fluorescence emission of the unique tryptophan residue (λex=295 nm) was also affected by pH. Figure 5(D) (inset) shows the shift in the maximum position from 320 nm (pH 7.8) to 340 nm (pH 4.0), with a sigmoid dependence on pH and a midpoint effect at pH 5.9. A similar variation in the F340/F321 intensity ratio is observed between pH 6 and pH 4.5 (midpoint at pH 5.8) and a slight decrease below pH 4.
Figure 5. Fluorescence emission spectra of AnxA13b as a function of pH.
Emission spectra at λex=275 nm (A) and λex=295 nm (B) at different pH values were registered at 20 °C; protein concentration was 0.05 mg/ml. Only representative spectra are shown. Fluorescence is expressed in arbitrary units (a.u.). Variations in the fluorescence intensity of tyrosine residues at 305 nm [F305 (Tyr); determined as described in the Experimental section] and in the F340/F321 fluorescence intensity ratio of Trp227 (λex=295 nm) with pH are shown in (C) and (D) respectively. The lower panel in (C) represents Rayleigh scattering at 275 nm (RS275) of AnxA13b (●) or buffer (△) at different pH values. The inset in (D) shows the variation in the tryptophan fluorescence maximum position with pH.
Protein aggregation was studied following Rayleigh scattering at 275 nm (Figure 5C, RS275). No significant increase in scattering was observed from pH 7.8 to 5.0. Thus the changes observed in the Trp227 environment at pH 6.5–4.5 are independent of protein aggregation. On the other hand, even though the increase in Rayleigh scattering is simultaneous with the initial increase in tyrosine fluorescence, at pH values below 3, no protein aggregation is detected. Consequently, the increase in tyrosine fluorescence below pH 4 could be the result of a partial protein unfolding (in agreement with CD spectra) and/or a decrease in static quenching due to the loss of negative charge of carboxy groups in the surroundings of tyrosine residues.
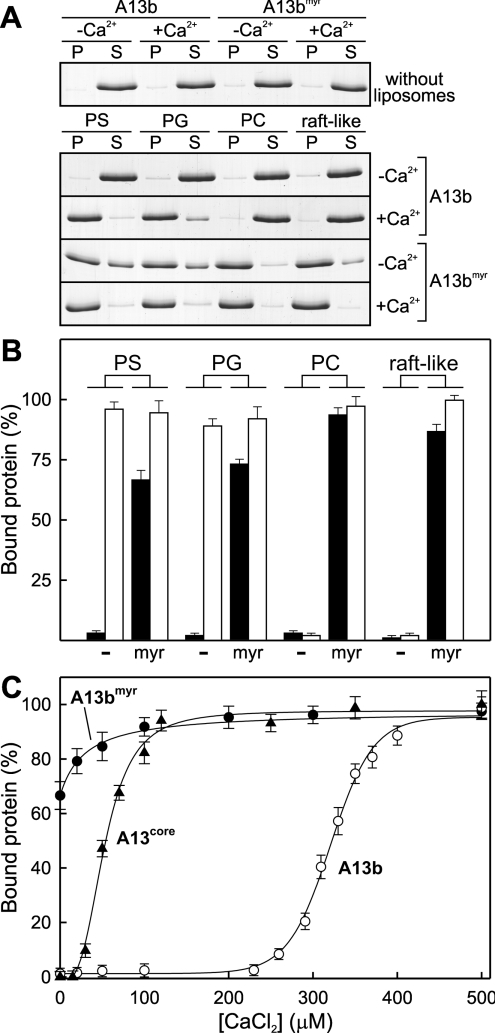
Phospholipid binding and vesicle aggregation
The binding of AnxA13b to phospholipid vesicles was studied by ultracentrifugation after incubation of the protein with an excess of large unilamellar vesicles (400 nm in diameter) in the absence (Figure 6B, black bars; 1 mM EGTA) or presence of 500 μM CaCl2 (Figure 6B, white bars). The vesicle pellets and supernatants were analysed by SDS/PAGE (Figure 6A). Figure 6(B) shows that the recombinant protein interacts in a calcium-dependent manner with liposomes composed of acidic phospholipids (PS and PG), but does not bind to PC or raft-like (PC/sphingomyelin/cholesterol, 1:1:1) vesicles. Figure 6(B) also shows the binding of myristoylated (radiolabelled) AnxA13bmyr to large unilamellar vesicles. The most interesting finding is that the myristoylated protein binds to PC and raft-like vesicles in a calcium-independent manner, whereas the non-myristoylated form is not able to bind either in the presence or absence of calcium. Additionally, 65–70% of the binding to acidic phospholipids is also achieved in the absence of calcium, and this process is favoured in the presence of calcium. The ability of A13bmyr to bind to membranes in the absence of calcium could explain its insolubility during the purification procedure.
Figure 6. Binding of AnxA13b to liposomes with different lipid composition.
The binding of AnxA13b to 400 nm unilamellar liposomes with different lipid composition [PS, PG, PC and PC/sphingomyelin/cholesterol, 1:1:1 (raft-like)] was performed by ultracentrifugation in the absence of calcium (1 mM EGTA; −Ca2+ and closed bars) or in the presence of 500 μM CaCl2 (+Ca2+ and open bars) with a lipid/protein molar ratio of 800:1. (A) AnxA13b in the pellets (P) and in the supernatants (S) was analysed by SDS/PAGE followed by Coomassie Blue staining; representative gels are given. (B) Quantification of the binding of non-myristoylated (−) and myristoylated (myr) AnxA13b was carried out by densitometry of the gels or by radioactivity measurement of myristoylated AnxA13b. (C) Influence of calcium concentration on the binding of non-myristoylated (A13b; ○) and myristoylated (A13bmyr; ●) AnxA13b, and its protein core (A13core; ▲) to 400 nm PS vesicles was analysed as in (A), but using CaCl2 concentrations ranging from 0 (1 mM EGTA) to 500 μM. Data in (B) and (C) represent means±S.D. for at least three independent experiments.
A more detailed analysis of the calcium dependence of AnxA13b, AnxA13bmyr and AnxA13core binding to PS vesicles is shown in Figure 6(C). The binding of AnxA13b follows a sigmoid pattern, with a midpoint at 321 μM CaCl2. Calcium requirements for binding of recombinant AnxA13b to PS vesicles are much higher than those described for any other analysed annexin [10]. Surprisingly, removal of the N-terminal extension decreases calcium requirements and AnxA13core shows a midpoint at 53 μM CaCl2, with a total binding at calcium concentrations over 120 μM (Figure 6C). The binding of AnxA13bmyr to PS vesicles is also enhanced by addition of calcium.
Binding of AnxA13b to PS has also been analysed by fluorescence emission spectroscopy using unilamellar vesicles (50 nm in diameter) with a constant lipid/protein molar ratio (800:1) and varying CaCl2 concentration (Figure 7). The global emission spectrum (λex=275 nm) after the addition of PS vesicles in the absence of calcium is quite similar to that registered in the absence of phospholipids, showing a maximum at 307 nm (Figure 7A); however, a slight shift in the Trp227 emission (λex=295 nm) is observed from 321 nm towards 326 nm as well as an approx. 25% decrease in the quantum yield (Figure 7B, continuous line). The sequential addition of calcium first shifts the Trp227 emission maximum towards 340 nm with a midpoint around 324 μM CaCl2 (Figures 7B and 7D, inset), and then induces an increase in the quantum yield that reaches a maximum at approx. 500 μM CaCl2 (Figure 7B). These changes are also reflected in a sigmoid increase in the F340/F326 fluorescence ratio that is slightly delayed in comparison with the changes detected in the maximum position (approx. 369 μM CaCl2; Figure 7D, inset). Interestingly, when tyrosine emission spectra are calculated by subtraction of the Trp227 contribution to the global spectra, a sigmoid decrease in the intensity at 305 nm is detected with a midpoint at 361 μM CaCl2 (Figure 7C).
Figure 7. Calcium-dependent changes in the fluorescence emission spectra of AnxA13b in the presence of PS liposomes.
Emission spectra at λex=275 nm (A) and λex=295 nm (B) in the absence of calcium or at increasing CaCl2 concentration were registered at 20 °C in the presence of 50 nm unilamellar PS vesicles using a lipid/protein molar ratio of 800:1 (protein concentration: 0.2 mg/ml or 5 μM). The broken line represents the spectra in the absence of calcium and PS. Only representative calcium concentrations and spectra are shown. Fluorescence is expressed in arbitrary units (a.u.). Variations in the fluorescence intensity of tyrosine residues at 305 nm [F305 (Tyr); determined as described in the Experimental section] and in the F340/F326 fluorescence intensity ratio of Trp227 (λex=295 nm) with calcium concentration are shown in (C) and (D) respectively. The inset in (D) shows the variation in the tryptophan fluorescence maximum position with calcium concentration.
Induction of vesicle aggregation was analysed using PS or PG vesicles at different protein and calcium concentrations. No aggregation was induced by the recombinant protein even at high protein (2 μM) or calcium (1 mM CaCl2) concentrations.
Changes in secondary structure after binding of AnxA13b to 50 nm unilamellar PS vesicles were studied by CD spectroscopy. In the presence of EGTA, the spectrum presents a different shape than in the absence of vesicles (Figures 2A and 2B, continuous lines); this change could be the result of either light scattering due to the presence of liposomes or an actual increase in α-helical content or length. In any case, addition of calcium (750 μM) induces a further spectral change (Figure 2B, broken line) and a great increase in the Tm (69 °C; Figure 2B, inset) that is neither induced by magnesium nor accompanied by an increase in light scattering. Taking into account that CCA does not predict significant changes in secondary-structure percentages, the changes in the CD spectrum after PS binding may correspond to an increase in the length of α-helices.
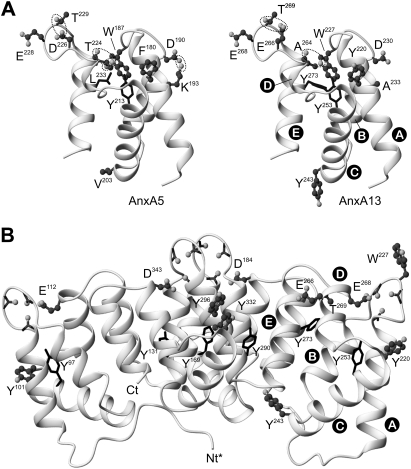
Structural model
We have developed a three-dimensional model of AnxA13b in the calcium-bound form and a model for domain III in the calcium-free conformation in order to analyse whether the stabilizing interactions and conformational changes described for other annexins, mainly AnxA5, have an equivalence in AnxA13b (Figure 8).
Figure 8. AnxA13b three-dimensional model.
Three-dimensional protein models of human AnxA13b were derived by sequence threading through X-ray crystallography co-ordinates of different annexins using the Swiss-Model Server version 3.5. (A) Comparison of the three-dimensional structure of domain III of human AnxA5 with that predicted for AnxA13 in the absence of calcium bound to this domain (closed conformation). Dotted lines mark hydrogen bonds or salt bridges essential for the maintenance of the closed calcium-free conformation. (B) Three-dimensional protein model of AnxA13b (residues 44–357) in the ‘high-calcium’ form. Nt*, residues 1–43 from the non-homologous N-terminal domain of AnxA13b are not shown. Ct, C-terminus. Tyrosine residues are represented in a wire-frame style if they are predicted as buried or in a ball-and-stick style if they are exposed to the solvent. The four major calcium co-ordination sites are located on the upper convex surface; the carbonyl groups and acid residues proposed to be involved in calcium co-ordination are shown together with some additional residues commented on in the text. Helix nomenclature in domain III is shown. Note that the orientation of domain III is not the same in (A) and (B) to facilitate the view of the stabilizing bonds in the closed conformation. The Figure was prepared using the MOLMOL program [48].
It has been described that the equilibrium between the ‘closed’ calcium-free conformation in domain III of AnxA5 and the ‘open’ conformation acquired when calcium is bound, depends strongly on a few specific interactions that stabilize the closed conformation: hydrogen bonds between Asp226 and Thr229, and between Thr224 and Trp187, and a salt bridge between Asp190 and Lys193 [27,33,34]. According to the sequence alignment between AnxA5 and AnxA13b, and taking into account the structural model proposed for the latter, AnxA13b can only establish hydrogen bonds between Glu266 and Thr269 and between the carbonyl oxygen of Ala264 and Trp227; AnxA13b presents alanine residues (Ala233 and Ala264) in positions equivalent to Lys193 and Thr224 in AnxA5, and thus some of these stabilizing interactions cannot be established (Figure 8A). The Figure also shows the four tyrosine residues (220 and 243, exposed, and 253 and 273, buried; according to Swiss PDB viewer version 3.7 prediction) present in domain III of AnxA13b and the residues in equivalent positions in AnxA5.
The only tryptophan residue in AnxA13b (Trp227) is located within the IIIA–IIIB loop, in an equivalent position to Trp187 in AnxA5, Trp186 in AnxA4 and Trp190 in AnxA3. The tryptophan residues in AnxA4 and AnxA5 (Figure 8A) lie inside the protein core in a hydrophobic environment, as has been deduced from the crystal structures obtained in the absence of calcium bound in domain III [4,35]. The predicted model for AnxA13b also locates Trp227 in a hydrophobic buried environment.
Figure 8(B) shows the protein model predicted for the calcium-bound conformation of AnxA13b. The conformational change upon calcium binding can be observed through comparison of domain III in Figures 8(A) and 8(B). The main changes affect the regions involved in type II calcium-binding sites: the AB loop, with exposure of Trp227, and helix D, which increases in length and positions Glu268 in the correct orientation to establish co-ordinations with calcium together with the carbonyl oxygens from Gly223, Arg226 and Gly228 (located in the AB loop). Exposed tyrosine residues are shown in a ‘ball-and-stick’ style, and buried ones in a ‘wire-frame’ style.
DISCUSSION
Analysis of the structure and function of AnxA13b is of great interest, as it is the founder member of the vertebrate family of annexins. It can be therefore considered as a prototype for conserved properties throughout the evolution of this superfamily of proteins [5,11]. Moreover, its specific pattern of expression and function in epithelial polarized cells presents some peculiarities in comparison with the rest of annexins, such as the binding to rafts [17–20]. In the present study, we wanted to gain insight into conformational changes induced by calcium binding, as well as by acidic pH, probably a shared characteristic among annexins. Additionally, we have studied the binding of AnxA13b, AnxA13bmyr and AnxA13core to phospholipid vesicles, which could help us to understand its physiological functions and the essential role of the N-terminal region.
The overall secondary structure of AnxA13b is very similar to that reported for other annexins with a medium/short N-terminus, in which the mainly α-helical protein core has been described as a quite stable arrangement. However, the Tm of AnxA13b (44 °C) is the lowest reported to date among vertebrate annexins, only comparable with that of AnxA11 (45 °C) [28]. Interestingly, AnxA13core presents a Tm value approx. 6 °C higher than AnxA13b and is quite similar to that described for AnxA5 [31]. The great degree of structural similarity found in the protein core of the ‘A’ family, and the results of the present study for the AnxA13core, indicate that differences in the N-terminus are probably the origin of the differences in thermal stability. In this case, the random-coil organization of this extension could induce a destabilization of the protein core, as we have previously reported for the long random coil N-terminus of AnxA11 [28]; AnxA1 and AnxA2, that possess N-termini similar in length to that of AnxA13b, but organized mainly in α-helical conformation, present a significantly higher Tm (approx. 60 °C in AnxA1) [36].
In contrast with the small effects that calcium binding seems to induce on the CD spectra, the Tm increases from 44 to approx. 55 °C when the calcium-binding sites in AnxA13b are saturated. This stabilization cannot be reproduced by the addition of magnesium. The Tm increase is similar to that observed for other annexins [31], and supports the notion that the interaction with calcium affects the whole protein core and is not limited to the binding sites, as suggested previously [32]. In any case, the calcium-bound conformation of AnxA13b shows a lower Tm than that of AnxA5 and AnxA11 (approx. 60 °C) [28,31].
AnxA13b presents only one tryptophan residue with a fluorescence emission maximum at 321 nm and barely accessible to acrylamide (KSV of 2.99 M−1). These data indicate that Trp227 is located in a hydrophobic cleft in the calcium-free conformation similar to Trp187 in AnxA5 [23,33,34] and in good accordance with the predicted three-dimensional model proposed for domain III of AnxA13b (Figure 8B). It has been reported that the hydrogen bond between Asp226 and Thr229 is essential for the stability of the closed conformation of domain III of AnxA5. This bond is broken after calcium binding or when pH is lowered with the consequent conformational rearrangement [33,34]. Interestingly, these residues have their equivalent in AnxA13b (Glu266 and Thr269) and thus this interaction could take place in AnxA13b (Figure 8A). Taking into account that AnxA13b has been proposed as the founder member of vertebrate annexins [5,11], the closed conformation in domain III could be a conserved configuration inherited from ancient annexins and the constitutively open conformation in other annexins, such as AnxA3 and AnxA8, may reflect its adaptation to specific functions [37].
Whereas calcium binding does not modify the overall secondary structure of AnxA13b in the absence of phospholipids, the fluorescence emission spectrum of Trp227 is greatly altered as a result of a conformational change that affects the IIIA–IIIB loop. These changes in the tryptophan environment take place at lower calcium concentrations than those required for thermal stabilization, suggesting that calcium-binding sites different from the type II also contribute to the stabilization of the AnxA13b protein core. The emission maximum red-shift of 23 nm reflects the movement from a hydrophobic to a polar environment. Accordingly, the quenching constant increases to 14.58 M−1, characteristic of an almost completely exposed tryptophan residue [38]. These observations can be explained by the rearrangement of the annexin fold, which only involves a minor modification in the overall secondary structure, but greatly alters the tryptophan environment, as described for AnxA5 [31,39]. However, the quantum yield of this residue is almost unaltered, even though the polar environment is a strong tryptophan quencher. Thus Trp187 is likely to be quenched already when it is buried in the hydrophobic cleft owing to the establishment of hydrogen bonds with Thr224 (Figure 8A) [34]. Even though AnxA13b does not present a threonine residue in this position (Ala246), quenching of Trp227 could originate from a hydrogen bond with the carbonyl group of Ala246, whose oxygen atom is close to the nitrogen in Trp227.
As mentioned previously, the predicted three-dimensional model and the sequence alignment between AnxA13b and AnxA5 indicate that the former annexin presents only some of the stabilizing bonds that maintain the closed conformation. It can be therefore suggested that the AnxA13b calcium-free conformation is not as stable as that in AnxA5. In fact, we have observed that acidification induces a gradual shift in the Trp227 environment similar to that described upon calcium binding, with a midpoint effect at pH 5.8–5.9, whereas AnxA5 shows this effect at a more acidic pH value [27,33]. The alteration in Trp227 down to pH 4.1 is only accompanied by small secondary-structure changes that mainly affect the length of α-helices. If this structural change is similar to that reported for AnxA5 after calcium binding, an increase in the length of helices D could take place [34].
It has been suggested that the conformation acquired by AnxA5 and AnxB12 at mildly acidic pH may be an intermediate state for their calcium-independent binding to membranes under these conditions [40–43]. The N-terminal half of AnxA6 also presents a conformational change at acidic pH, but it involves a partial denaturation of the protein core with exposure of hydrophobic regions [44] that is not detected in annexins A5, A13b and B12. Moreover, site-directed spin labelling has revealed that AnxB12 inserts into the lipid bilayer after undergoing a profound structural reorganization in which the conformation described at mildly acidic pH may be an intermediate state [41,43,45]. This conserved characteristic between Hydra and vertebrate annexins could be a consequence of convergent evolution or could well be a common ancestral property of all annexins. AnxA13b (more related to non-vertebrate and ancient annexins) shows this acidic pH-triggered rearrangement in solution, which strongly argues for the latter assumption. As a whole, these observations suggest the presence of a proton-dependent switch in annexins which harbours the information to induce membrane insertion. This insertion could explain some of the physiological properties of these proteins, such as calcium channel activity, and could also underlie its pathway of secretion.
We have found that AnxA13b maintains the phospholipid specificity, but requires much higher calcium concentrations than those described for the rest of the annexins [1–3,9]. This finding is quite surprising considering that the more calcium accessible open conformation should be favoured in the proximity of anionic phospholipids (local pH decrease of approx. 1.6 units [46]) and that the annexin core is highly homologous. Thus changes in the protein sequence are not likely to be responsible for this low affinity. In consequence, the relatively long AnxA13b N-terminus could interact with the protein core, inducing both the loss in stability (low Tm) and the high calcium requirement for binding to liposomes. This hypothesis is strongly supported by the fact that truncated AnxA13core not only presents significantly higher thermal stability than the intact molecule, but also has much lower calcium requirements for binding to PS vesicles.
Addition of PS vesicles to AnxA13b in the absence of calcium induces an alteration in the fluorescence emission spectrum of Trp227 and in the CD spectrum similar to those observed when the pH is lowered. This change could be explained by the already mentioned effect of anionic phospholipids in the pH near the surface of the membrane [46]. Subsequent addition of calcium initially induces a red-shift in the fluorescence maximum, with a midpoint effect at the same calcium concentration required for 50% binding, followed by an increase in the Trp227 quantum yield. The requirement for an initial nucleation step could be responsible for this sequential modification of the spectrum. Additionally, we have observed by CD spectroscopy that binding of AnxA13b to PS liposomes induces a structural rearrangement, which mainly affects the length of α-helices, that is accompanied by a large increase (approx. 25 °C) in the Tm, in a similar pattern to what we have reported previously for AnxA11 [28].
Regarding the variation in tyrosine emission spectra with calcium concentration in the presence of PS, a decrease in the quantum yield of these residues is observed almost in parallel with the alteration in the tryptophan residue. This sigmoid decrease is not observed in the absence of PS or when the pH is decreased. Thus it is highly probable that this alteration in tyrosine fluorescence is not directly due to the conformational change that takes place upon calcium binding (open conformation), but is more probably due to the establishment of interactions between annexin molecules on the surface of the liposomes. In fact, according to the model proposed in Figure 8(B), Tyr101 and Tyr220 are highly exposed residues located in regions equivalent to those described in AnxA5 to play a key role in its self-assembly on the surface of the membranes [47]. Taking into account the similarities between these two annexins, the mentioned tyrosine residues would change to a less hydrophilic environment, probably forming hydrogen bonds that are responsible for the static quenching of the fluorescence emission.
The high calcium concentrations required for the binding of recombinant AnxA13b to membranes are never achieved under physiological conditions inside the cell. In addition, we have only detected calcium-dependent binding of AnxA13b to acidic phospholipids (PS and PG), but not to neutral ones (PC) or to raft-like liposomes. On the other hand, it has been described that this protein binds to rafts in vivo, and that this interaction is essential for its functionality [19,20]. The only difference between recombinant and wild-type AnxA13b is N-myristoylation; therefore this post-translational modification must be responsible for binding to lipid rafts. AnxA13bmyr binds in a calcium-independent manner to acidic phospholipids (more than 70%) and to PC and raft-like liposomes, confirming the essential role of N-terminal myristoylation of this annexin for its in vivo function. Even though it has been reported that AnxA13a and AnxA13b bind in a calcium-independent manner to membranes, and that myristoylation is required for this binding [20], these studies were performed using membrane extracts from cells transfected with wild-type and non-myristoylated mutants of these proteins. Thus this is the first time that a direct interaction between myristoylated AnxA13b and neutral and raft-like structures has been shown, without disregarding that other proteins may modulate this interaction.
We have performed for the first time an exhaustive spectroscopical/structural characterization of AnxA13b, the oldest vertebrate annexin with the most tissue-specific expression. This ancient annexin, more related to invertebrate or even protist and plant annexins, presents some structural features that have been conserved during the evolution of vertebrate annexins. Thus forces stabilizing the closed calcium-free conformation, the structural changes upon calcium binding and the acidic pH conformational switch detected in quite modern annexins (such as AnxA5) are likely to be conserved properties that are inherited from ancestral annexins and are not the result of a convergent evolution. On the other hand, it is noteworthy that the N-terminus of this annexin presents unique properties, not only because of alternative splicing that yields two isoforms with different functionality regarding membrane trafficking, but also because it is N-myristoylated. This post-translational modification probably alters the structure and stability of the highly conserved protein core, as we have observed that the non-myristoylated protein is relatively unstable compared with its protein core and with other vertebrate annexins. However, the most surprising finding has been the high calcium requirements that the non-myristoylated protein presents; this impairment disappears upon N-myristoylation. This unique structural feature enables AnxA13b to interact directly, in a calcium-independent manner, with raft-like domains, an interaction that is essential for its in vivo functionality.
Acknowledgments
This work was supported by the Spanish grant BMC2002-01407 and BMC2002-00827 from Dirección General de Investigación. A.G.-A. has been recipient of the Spanish fellowship Severo Ochoa from the Ferrer Research Group.
References
- 1.Raynal P., Pollard H. B. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim. Biophys. Acta. 1994;1197:63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 2.Swairjo M. A., Seaton B. A. Annexin structure and membrane interactions: a molecular perspective. Annu. Rev. Biophys. Struct. 1994;23:193–213. doi: 10.1146/annurev.bb.23.060194.001205. [DOI] [PubMed] [Google Scholar]
- 3.Gerke V., Moss S. E. Annexins: from structure to function. Physiol. Rev. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 4.Liemann S., Huber R. Three-dimensional structure of annexins. Cell. Mol. Life Sci. 1997;53:516–521. doi: 10.1007/s000180050065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez M. P., Morgan R. O. Structure, function and evolution of the annexin supergene family. In: Bandorowicz-Pikula J., editor. Annexins: Biological Importance and Annexin-Related Pathologies. Georgetown: Landes Bioscience; 2003. pp. 21–37. [Google Scholar]
- 6.Huber R., Römisch J., Pâques E. P. The crystal and molecular structure of human annexin V, an anticoagulant protein that binds to calcium and membranes. EMBO J. 1990;9:3867–3873. doi: 10.1002/j.1460-2075.1990.tb07605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel D. R., Jao C. C., Mailliard W. S., Isas J. M., Langen R., Haigler H. T. Calcium-dependent binding of annexin 12 to phospholipid bilayers: stoichiometry and implications. Biochemistry. 2001;40:7054–7060. doi: 10.1021/bi010345+. [DOI] [PubMed] [Google Scholar]
- 8.Montaville P., Neumann J., Russo-Marie F., Oschenbein F., Saison A. A new consensus sequence for phosphatidylserine recognition by annexins. J. Biol. Chem. 2002;277:24684–24693. doi: 10.1074/jbc.M109595200. [DOI] [PubMed] [Google Scholar]
- 9.Turnay J., Guzmán-Aránguez A., Lecona E., Pérez-Ramos P., Fernández-Lizarbe S., Olmo N., Lizarbe M. A. Influence of the N-terminal domain of annexins in their functional properties. Recent Res. Dev. Biochem. 2003;4:53–78. [Google Scholar]
- 10.Turnay J., Lecona E., Guzmán-Aránguez A., Pérez-Ramos P., Fernández-Lizarbe S., Olmo N., Lizarbe M. A. Annexins: structural characteristics of the N-terminus and influence on the overall structure of the protein. Recent Res. Dev. Biochem. 2003;4:79–95. [Google Scholar]
- 11.Morgan R. O., Fernandez M. P. Distinct annexin subfamilies in plants and protists diverged prior to animal annexins and from a common ancestor. J. Mol. Evol. 1997;44:178–188. doi: 10.1007/pl00006134. [DOI] [PubMed] [Google Scholar]
- 12.Morgan R. O., Bell D. W., Testa J. R., Fernandez M. P. Genomic locations of ANX11 and ANX13 and the evolutionary genetics of human annexins. Genomics. 1998;48:100–110. doi: 10.1006/geno.1997.5148. [DOI] [PubMed] [Google Scholar]
- 13.Bances P., Fernández M. R., Rodríguez-García M. I., Morgan R. O., Fernandez M. P. Annexin A11 (ANXA11) gene structure as the progenitor of paralogous annexins and source of orthologous cDNA isoforms. Genomics. 2000;69:95–103. doi: 10.1006/geno.2000.6309. [DOI] [PubMed] [Google Scholar]
- 14.Iglesias J. M., Morgan R. O., Jenkins N. A., Copeland N. G., Gilbert D. J., Fernandez M. P. Comparative genetics and evolution of annexin A13 as the founder gene of vertebrate annexins. Mol. Biol. Evol. 2002;19:608–618. doi: 10.1093/oxfordjournals.molbev.a004120. [DOI] [PubMed] [Google Scholar]
- 15.Wice B. M., Gordon J. I. A strategy for isolation of cDNAs encoding proteins affecting human intestinal epithelial cell growth and differentiation: characterization of a novel gut-specific N-myristoylated annexin. J. Cell Biol. 1992;116:405–422. doi: 10.1083/jcb.116.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiedler K., Lafont F., Parton R. G., Simons K. Annexin XIIIb: a novel epithelial specific annexin is implicated in vesicular traffic to the apical plasma membrane. J. Cell Biol. 1995;128:1043–1053. doi: 10.1083/jcb.128.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massey-Harroche D., Mayran N., Maroux S. Polarized localizations of annexins I, II, VI and XIII in epithelial cells of intestinal, hepatic and pancreatic tissues. J. Cell Sci. 1998;111:3007–3015. doi: 10.1242/jcs.111.20.3007. [DOI] [PubMed] [Google Scholar]
- 18.Massey-Harroche D. Epithelial cell polarity as reflected in enterocytes. Microsc. Res. Tech. 2000;49:353–362. doi: 10.1002/(SICI)1097-0029(20000515)49:4<353::AID-JEMT4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Lafont F., Lecat S., Verkade P., Simons K. Annexin XIIIb associates with lipid microdomains to function in apical delivery. J. Cell Biol. 1998;142:1413–1427. doi: 10.1083/jcb.142.6.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lecat S., Verkade P., Thiele C., Fiedler K., Simons K., Lafont F. Different properties of two isoforms of annexin XIII in MDCK cells. J. Cell Sci. 2000;113:2607–2618. doi: 10.1242/jcs.113.14.2607. [DOI] [PubMed] [Google Scholar]
- 21.Noda Y., Okada Y., Saito N., Setou M., Xu Y., Zheizeng Z., Hirokawa N. KIFC3, a microtubule minus end-directed motor for the apical transport of annexin XIIIb-associated Triton-insoluble membranes. J. Cell Biol. 2001;155:77–88. doi: 10.1083/jcb.200108042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plant P. J., Lafont F., Lecat S., Verkade P., Simons K., Rotin D. Apical membrane targeting of Nedd4 is mediated by an association of its C2 domain with annexin XIIIb. J. Cell Biol. 2000;149:1473–1483. doi: 10.1083/jcb.149.7.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arboledas D., Olmo N., Lizarbe M. A., Turnay J. Role of the N-terminus in the structure and stability of chicken annexin V. FEBS Lett. 1997;416:217–220. doi: 10.1016/s0014-5793(97)01207-6. [DOI] [PubMed] [Google Scholar]
- 24.Duronio R. J., Jackson-Machelski E., Heuckeroth R. O., Olins P. O., Devine C. S., Yonemoto W., Slice L. W., Taylor S. S., Gordon J. I. Protein N-myristoylation in Escherichia coli: reconstitution of a eukaryotic protein modification in bacteria. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1506–1510. doi: 10.1073/pnas.87.4.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gómez-Gutiérrez J., Rodríguez-Crespo I., Peterson D. L., Gavilanes F. Reconstitution of hepatitis B surface antigen proteins into phospholipid vesicles. Biochim. Biophys. Acta. 1994;1192:45–52. doi: 10.1016/0005-2736(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 26.Perczel A., Park K., Fasman G. D. Analysis of the circular dichroism spectrum of proteins using the convex constraint algorithm: a practical guide. Anal. Biochem. 1992;203:83–93. doi: 10.1016/0003-2697(92)90046-a. [DOI] [PubMed] [Google Scholar]
- 27.Beermann ofm cap B. B., Hinz H.-J., Hofmann A., Huber R. Acid induced equilibrium unfolding of annexin V wild type shows two intermediate states. FEBS Lett. 1998;423:265–269. doi: 10.1016/s0014-5793(98)00105-7. [DOI] [PubMed] [Google Scholar]
- 28.Lecona E., Turnay J., Olmo N., Guzmán-Aránguez A., Morgan R. O., Fernandez M. P., Lizarbe M. A. Structural and functional characterization of recombinant mouse annexin A11: influence of calcium binding. Biochem. J. 2003;373:437–449. doi: 10.1042/BJ20021721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 30.Campos B., Mo Y. D., Mealy T. R., Li C. W., Swairjo M. A., Balch C., Head J. F., Retzinger G., Dedman J. R., Seaton B. A. Mutational and crystallographic analyses of interfacial residues in annexin V suggest direct interactions with phospholipid membrane components. Biochemistry. 1998;37:8004–8010. doi: 10.1021/bi973142n. [DOI] [PubMed] [Google Scholar]
- 31.Turnay J., Olmo N., Gasset M., Iloro I., Arrondo J. L. R., Lizarbe M. A. Calcium-dependent conformational rearrangements and protein stability in chicken annexin A5. Biophys. J. 2002;83:2280–2291. doi: 10.1016/s0006-3495(02)73988-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ayala-Sanmartin J., Vincent M., Sopkova J., Gallay J. Modulation by Ca2+ and by membrane binding of the dynamics of domain III of annexin 2 (p36) and the annexin 2–p11 complex (p90): implications for their biochemical properties. Biochemistry. 2000;39:15179–15189. doi: 10.1021/bi000501x. [DOI] [PubMed] [Google Scholar]
- 33.Sopkova J., Vincent M., Takahashi M., Lewit-Bentley A., Gallay J. Conformational flexibility of domain III of annexin V studied by fluorescence of tryptophan 187 and circular dichroism: the effect of pH. Biochemistry. 1998;37:11962–11970. doi: 10.1021/bi980773o. [DOI] [PubMed] [Google Scholar]
- 34.Sopková-De Oliveira Santos J., Vincent M., Tabaries S., Chevalier A., Kerboeuf D., Russo-Marie F., Lewit-Bentley A., Gallay J. Annexin A5 D226K structure and dynamics: identification of a molecular switch for the large-scale conformational change of domain III. FEBS Lett. 2001;493:122–128. doi: 10.1016/s0014-5793(01)02285-2. [DOI] [PubMed] [Google Scholar]
- 35.Zanotti G., Malpeli G., Gliubich F., Folli C., Stoppini M., Olivi L., Savoia A., Berni R. Structure of the trigonal crystal form of bovine annexin IV. Biochem. J. 1998;329:101–106. doi: 10.1042/bj3290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosengarth A., Rösgen J., Hinz H.-J., Gerke V. A comparison of the energetics of annexin I and annexin V.J. Mol. Biol. 1999;288:1013–1025. doi: 10.1006/jmbi.1999.2732. [DOI] [PubMed] [Google Scholar]
- 37.Réty S., Sopková-de Oliveira Santos J., Dreyfuss L., Blondeau K., Hofbaurová K., Raguénès-Nicol C., Kerbouef D., Renouard M., Russo-Marie F., Lewitt-Bentley A. The crystal structure of annexin A8 is similar to that of annexin A3. J. Mol. Biol. 2005;345:1131–1139.. doi: 10.1016/j.jmb.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 38.Lakowicz J. R. Quenching of fluorescence. In: Lakowicz J. R., editor. Principles of Fluorescence Spectroscopy. 2nd edn. New York: Kluwer Academic/Plenum Publishers; 1999. pp. 237–265. [Google Scholar]
- 39.Sopkova J., Gallay J., Vincent M., Pancoska P., Lewit-Bentley A. The dynamic behavior of annexin V as a function of calcium ion binding: a circular dichroism, UV absorption, and steady-state and time-resolved fluorescence study. Biochemistry. 1994;33:4490–4499. doi: 10.1021/bi00181a008. [DOI] [PubMed] [Google Scholar]
- 40.Kohler G., Hering U., Zschoring O., Arnold K. Annexin V interaction with phosphatidylserine-containing vesicles at low and neutral pH. Biochemistry. 1997;36:8189–8194. doi: 10.1021/bi9703960. [DOI] [PubMed] [Google Scholar]
- 41.Langen R., Isas J. M., Hubbell W. L., Haigler H. T. A transmembrane form of annexin XII detected by site-directed spin labeling. Proc. Natl. Acad. Sci. U.S.A. 1998;95:14060–14065. doi: 10.1073/pnas.95.24.14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isas J. M., Cartailler J. P., Sokolov Y., Patel D. R., Langen R., Luecke H., Hall J. E., Haigler H. T. Annexins V and XII insert into bilayers at mildly acidic pH and form ion channels. Biochemistry. 2000;39:3015–3022. doi: 10.1021/bi9922401. [DOI] [PubMed] [Google Scholar]
- 43.Ladokhin A. S., Haigler H. T. Reversible transition between the surface trimer and membrane-inserted monomer of annexin 12. Biochemistry. 2005;44:3402–3409. doi: 10.1021/bi047805u. [DOI] [PubMed] [Google Scholar]
- 44.Golczak M., Kirilenko A., Bandorowicz-Pikula J., Desbat B., Pikula S. Structure of human annexin A6 at the air–water interface and in a membrane-bound state. Biophys. J. 2004;87:1215–1226. doi: 10.1529/biophysj.103.038240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isas J. M., Patel D. R., Jao C., Jayasinghe S., Cartailler J., Haigler H. T., Langen R. Global structural changes in annexin 12: the roles of phospholipid, Ca2+, and pH. J. Biol. Chem. 2003;278:30227–30234. doi: 10.1074/jbc.M301228200. [DOI] [PubMed] [Google Scholar]
- 46.Van der Goot F. G., González-Mañas J. M., Lakey J. H., Pattus F. A ‘molten-globule’ membrane-insertion intermediate of the pore-forming domain of colicin A. Nature (London) 1991;354:408–410. doi: 10.1038/354408a0. [DOI] [PubMed] [Google Scholar]
- 47.Oling F., Bergsma-Schutter W., Brisson A. Trimers, dimers of trimers, and trimers of trimers are common building blocks of annexin A5 two-dimensional crystals. J. Struct. Biol. 2001;133:55–63. doi: 10.1006/jsbi.2000.4337. [DOI] [PubMed] [Google Scholar]
- 48.Koradi R., Billeter M., Wüthrich K. MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graphics. 1996;14:51–55. doi: 10.1016/0263-7855(96)00009-4. [DOI] [PubMed] [Google Scholar]